FIGURE 1.
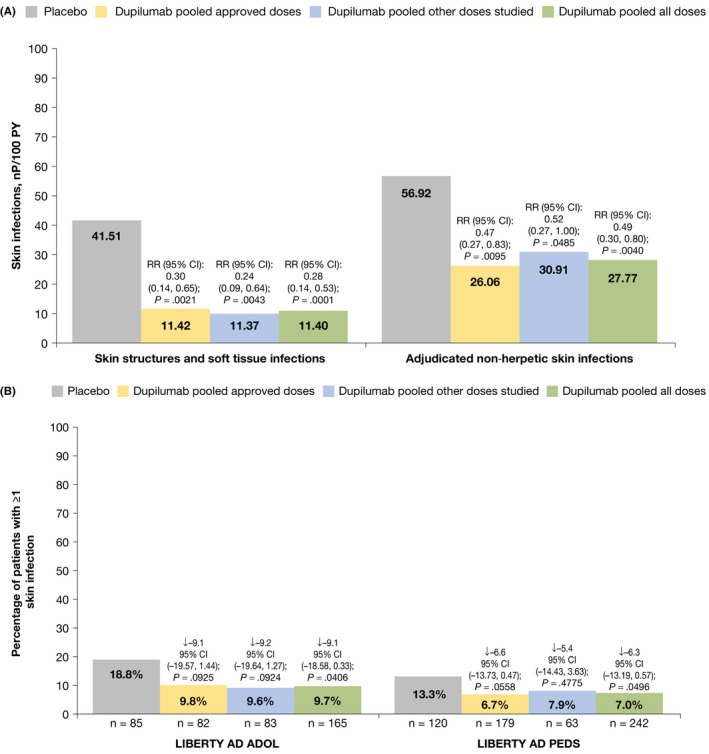
Exposure‐adjusted numbers of patients with treatment‐emergent skin infections (non‐herpetic) during the study treatment period. (A) Skin infections by HLT and adjudicated skin infections. (B) Proportion of patients having at least 1 skin infection treatment‐emergent adverse event (excluding herpetic infections) through week 16, by study. HLT selected from records of adjudicated skin infections excluding herpetic infections. ↓ = Difference versus placebo CI calculated using normal approximation. p‐values were derived by Cochran‐Mantel‐Haenszel (CMH) test stratified by baseline disease severity (IGA = 3 vs. IGA = 4) and baseline weight group (<60 kg vs. ≥60 kg) for study LIBERTY AD ADOL; by region (North America vs. Europe) and baseline weight group (<30 kg vs. ≥30 kg) for study LIBERTY AD PEDS. CI, confidence interval; HLT, MedDRA high‐level term; IGA, Investigator's Global Assessment; MedDRA, Medical Dictionary for Regulatory Activities; nP, number of patients with ≥1 event; PY, patient‐years; RR, risk ratio
