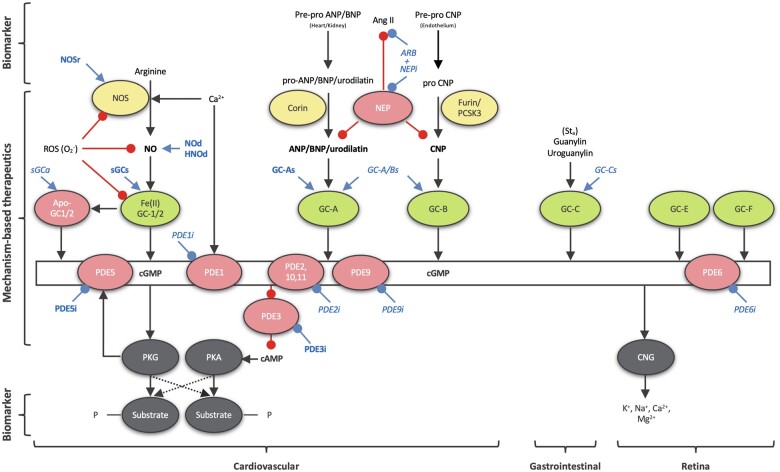
Figure 1.
Classical, curated representation of cGMP signalling. Shown in green are the GCs and in yellow their positive regulators; NO produced by NOS for soluble GC and NPs (ANP, BNP, and CNP) for particulate GC. Negative regulators (cGMP metabolizing PDEs 1, 2, 5, 6, 9, 10, 11, and NPs degrading NEP) and pathophysiological conditions (oxidized/heme-free apo-sGC) are shown in pink. All clinically relevant cGMP-modulating drugs are shown in blue (bold for approved drugs and in italic for drugs under investigation): ARB, Angiotensin II receptor blockers; GC-As, GC-A stimulators; GC-A/Bs, GC-A/B stimulators; NEPi, neprilysin inhibitors; NOd, NO donors; NOSr, NOS recoupling nutraceuticals; sGCa, sGC activators; sGCs, sGC stimulators; PDEi, PDE inhibitors. cGMP effector proteins (PKG and cyclic nucleotide-gated ion channels, CNG) and their substrates are shown in grey. cGMP can also inhibit some isoforms of the PDE enzyme family. In turn, this leads to an altered phosphoprotein profile, a decrease in intracellular calcium levels and sensitivity, and altered cGMP and cAMP levels (proffering crosstalk between cGMP and cAMP networks). GC-C is localized in intestines and GC-E and -F in the retina, thus not relevant to the context of this review. Ang II, angiotensin II; Sta, heat-stable enterotoxin I STa.