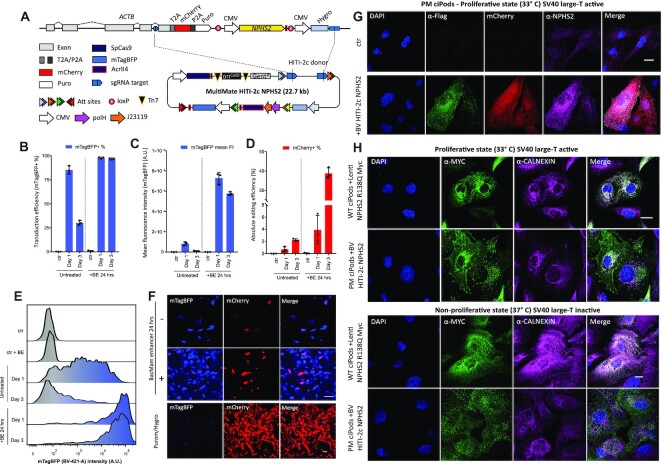
Figure 4.
Safe-harbour large cargo integration of NPHS2 into patient-derived R138Q mutant podocin podocytes. (A) Safe harbour ACTB-NPHS2-Myc-Flag strategy. ACTB C-terminal exon is replaced with a P2A mCherry T2A Puro tagged synthetic exon (5′ integration marker), followed by CMV NPHS2-Myc-Flag and CMV Hygro (3′ integration marker). polH VSV-G and CMV mTagBFP are included in vector design to pseudotype and monitor viral transduction, respectively. (B–E) PM ciPods transduced with BV MultiMate HITI-2c NPHS2 in presence/absence of 24 h BacMam enhancer treatment analysed at 24 or 72 h post transduction. (B) transduction efficiencies (mTagBFP+ %); (C) mTagBFP mean fluorescence intensity levels, (D) absolute gene editing efficiencies (mCherry+ %). In (B–E) histograms represents means of flow-cytometry data, error bars are standard deviations of n = 3 independent replicates. (E) representative flow-cytometry histogram of mTagBFP intensity relative to (C). (F) Widefield microscopy images of PM ciPods at 24 h post-transduction in presence/absence of BacMam enhancer (top panel) or 36 days post-transduction following Puromycin/Hygromycin selection (bottom panel). (G) Immunofluorescence of unselected PM ciPods transduce with BV MultiMate HITI-2c NPHS2. Successfully edited (mCherry+) cells, display correct expression of NPHS2 through either α-Flag or α-NPHS2 antibodies. DAPI is used to counterstain nuclei. Scalebar = 20 μm. (H) Comparison of engineered PM ciPods with WT CiPods overexpressing NPHS2 R138Q under proliferative (33°C, top panel) or non-proliferative (10 days at 37°C, bottom panel) culturing conditions. α-Calnexin labels the endoplasmic reticulum, α-Myc is used to stain NPHS2. Scalebar = 20 μm.