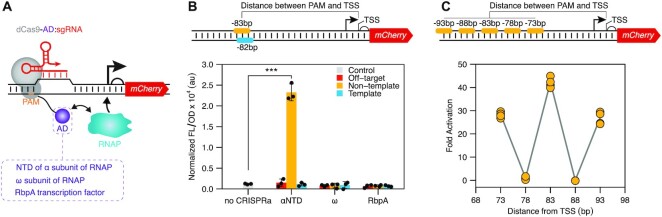
Figure 2.
Creating a CRISPR activation (CRISPRa) for Streptomyces venezuelae. (A) Schematic of CRISPRa mechanism. An activator domain (AD, colored purple) is translationally fused to a dCas9 via a flexible linker. The CRISPRa complex binds upstream of a target promoter to recruit RNA Polymerase (RNAP) and activate transcription of the target gene. (B) The N-terminal domain of the α subunit of RNAP (αNTD) can serve as an AD for CRISPRa in Streptomyces. Schematic of sgRNA binding sites used that target the non-template (NT) and template (T) strand upstream of a promoter driving mCherry expression. The indicated distances reflect the number of nucleotides intervening between the 5’ end of the PAM (not included) and the TSS (also not included). Fluorescence characterization of S. venezuelae cells conjugated with CRISPRa plasmid variants using different AD. Statistical significance was calculated using two-tailed unpaired Welch’s t-test. Statistically significant differences compared to the no-CRISPRi are shown as asterisks (***P-value < 0.005). (C) CRISPRa activation shows periodical distance-dependent activation patterns. Schematic of sgRNA binding sites used that target different sites on the non-template strand upstream of a promoter driving mCherry expression. Fluorescence characterization was performed by bulk fluorescence measurements (measured in units of fluorescence [FL]/optical density [OD] at 600 nm). Fold activation was calculated by dividing the [FL]/[OD] obtained in the presence of a CRISPRa against the no-CRISPRa control within each reporter plasmid. Data are reported as individual replicates with a line connecting the mean of each condition.