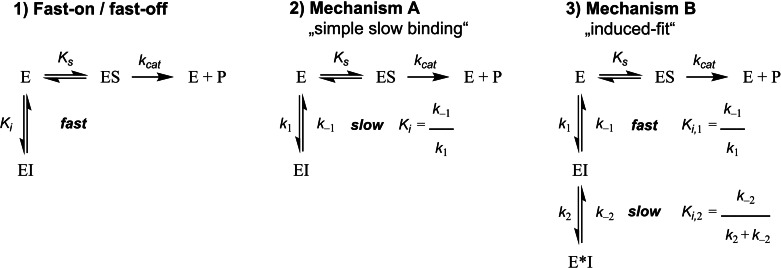
Figure 5.
Representative examples of different kinetic mechanisms of enzyme inhibition, including the relationships between the respective association and dissociation rate constants (e. g., k 1 & k −1) and the related equilibrium dissociation constant Ki . A) Fast‐on/fast‐off binding kinetics. For competitive fast‐on/fast‐off inhibitors the half maximal inhibitory concentration (IC50) and the Ki are directly related by the Cheng‐Prusoff equation; [34] B) slow‐binding Mechanism I: single‐step slow‐binding, k 1 & k −1 are inherently slow; C) slow‐binding Mechanism II: two‐step slow‐binding or “induced‐fit”. Initially, inhibitor and enzyme form an encounter complex [EI] that subsequently slowly undergoes isomerization to a binary enzyme inhibitor complex [E*I].