FIG 1.
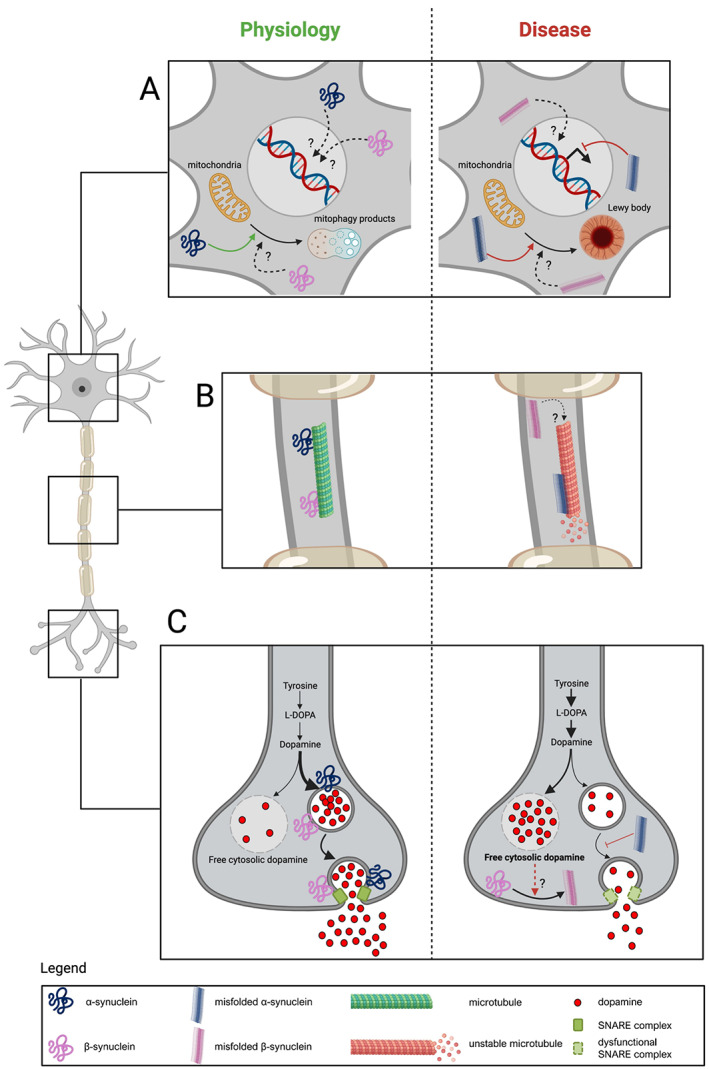
Main pathophysiological roles of α‐synuclein (blue) and β‐synuclein (purple) in neurons. (A) In the neuronal body, α‐synuclein participates in mitochondrial functioning and motility, contributing to redirecting damaged mitochondria to mitophagy. When this pathway is impaired, misfolded α‐synuclein may induce accumulation of dysmorphic organelles (ie, mitochondria) and formation of neuronal inclusions. The physiological impact of α‐synuclein on nuclear functions is unclear, whereas pathological α‐synuclein might be related to alterations in the transcription process. To what extent β‐synuclein and misfolded β‐synuclein affect neuronal organelles and nuclei is currently unknown. (B) α‐Synuclein interacts with several cytoskeletal components, among which the axonal microtubules are destabilized by misfolded and aggregated α‐synuclein. It is unknown whether β‐synuclein interacts with microtubules and other structural proteins. (C) The most relevant functions of α‐synuclein are exerted in presynaptic terminals and mainly deal with regulation of vesicular trafficking and homeostasis of neurotransmitters in both dopaminergic neurons and other cell types. Physiologically, α‐synuclein promotes storage of dopamine into presynaptic vesicles and its release through exocytosis. In synucleinopathies, the synthesis of dopamine might be overstimulated, resulting in dopamine‐induced neurotoxicity. β‐Synuclein may act as a synaptic chaperone and, hypothetically, its aggregation might be promoted by dopamine dyshomeostasis, thus contributing to neuronal damage. L‐DOPA, levodopa; SNARE, soluble N‐ethylmaleimide‐sensitive factor attachment proteins receptors. [Color figure can be viewed at wileyonlinelibrary.com]
