FIGURE 1.
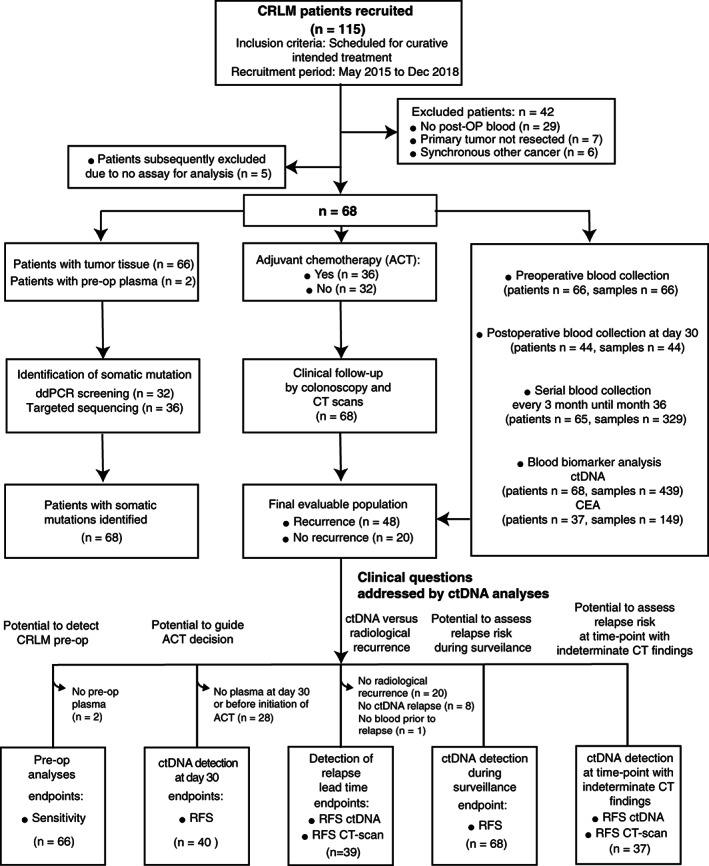
Patient enrollment, sample collection and definition of the patient subgroups used to address the defined clinical questions. N = 29: No post‐OP blood (The patients did not provide any blood samples after CRLM resection), N = 7: Primary tumor not resected (the scheduled resection of the primary cancer was canceled), N = 6: Synchronous other cancer, N = 5: No assay for analysis. ACT, adjuvant chemotherapy; ctDNA, circulating tumor DNA; CT, computed tomography; post‐op, postoperative; RFS, recurrence free survival
