Figure 1.
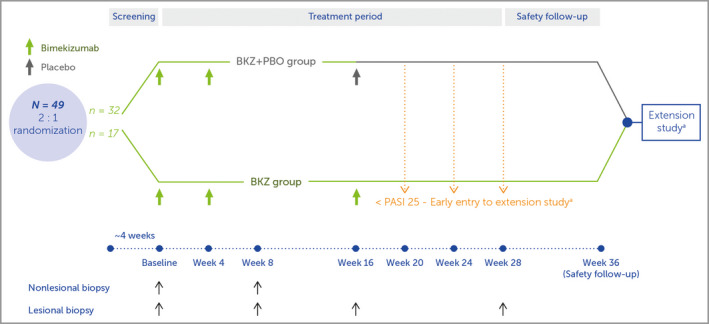
Study design. Study visits occurred at week 0 (baseline), and weeks 2, 4, 8, 12, 16, 20, 28 and 36 (safety follow‐up). The bimekizumab plus placebo (BKZ+PBO) group received two doses of bimekizumab 320 mg, at weeks 0 and 4, with placebo at week 16; the BKZ group received three doses of bimekizumab 320 mg, at weeks 0, 4 and 16. aOnly patients who achieved a 25% improvement from baseline Psoriasis Area and Severity Index score (PASI 25) in the first 16 weeks were eligible for entry to the extension study (NCT03230292) after the safety follow‐up visit; if these patients failed to achieve PASI 25 at weeks 20, 24 or 28 they were permitted to enter the extension study early, receiving the first dose of study drug at that visit.
