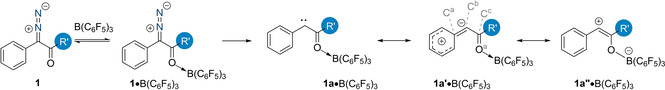
Table 2.
Calculated free energies for uncatalyzed carbene formation with different R′ groups, free energy difference between B(C6F5)3 catalyzed and uncatalyzed carbene formation, and free energy values for B(C6F5)3 catalyzed carbene formation. Free energies are given in kcal/mol. The B−O bond distance (rB−O) in 1 a ⋅ B(C6F5)3 .
|
| ||||||||
|---|---|---|---|---|---|---|---|---|
|
Entry |
R′ |
ΔG≠ 3 |
ΔG3 |
ΔG≠ 4 |
ΔG4 |
rB−O [Å] |
ΔΔG≠ 2=ΔG≠ 3–ΔG≠ 4 |
ΔΔG2=ΔG3–ΔG4 |
|
|
|
Uncatalyzed |
B(C6F5)3 catalyzed |
Catalyst efficiency [a] |
||||
|
1 |
Me |
30.4 |
10.4 |
9.5 |
−6.4 |
1.509 |
20.9 |
16.8 |
|
2 |
H |
31.3 |
13.4 |
10.5 |
−6.8 |
1.512 |
20.8 |
20.2 |
|
3 |
Ph |
31.2 |
9.3 |
11.4 |
−6.1 |
1.518 |
19.8 |
15.4 |
|
4 |
OMe |
32.0 |
13.1 |
25.9 |
6.5 |
1.533 |
6.1 |
6.6 |
|
5 |
OH |
30.6 |
12.1 |
26.1 |
7.8 |
1.534 |
4.5 |
4.3 |
|
6 |
F |
30.1 |
11.1 |
27.3 |
9.0 |
1.543 |
2.8 |
2.1 |
[a] Ability of the catalyst to reduce the activation free energies. The large values for ΔΔG≠ 2/ΔΔG2 indicate a high efficiency of the catalyst while the small values indicate a low efficiency of the catalyst.