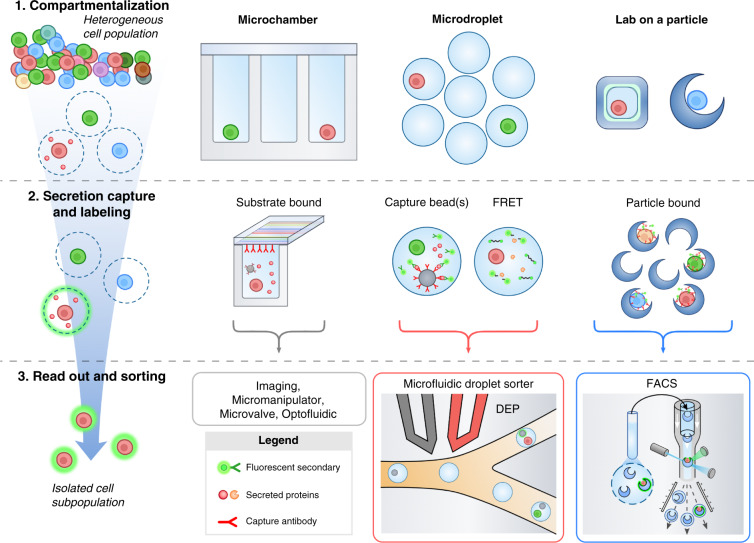
Fig. 2. Three Fundamental Steps for Single-Cell Secretion Screening.
The analysis of secreted products from individual cells relies on three fundamental processes. 1 Cells are compartmentalized into individual containers so that their secreted products are concentrated. In these compartments, the transport of secretions to neighboring cells, which leads to crosstalk, is reduced. This can be accomplished using microfabricated compartments, aqueous emulsions, or particle-based confinement approaches. 2 Secreted molecules accumulate and are converted into detectable signals. One common method to accumulate and label secretions is to use a sandwich of affinity capture elements such as antibodies. Capture antibodies concentrate secretions onto a solid phase, such as glass slides or capture beads, where they can be detected with secondary fluorescently labeled reporter antibodies. For bioactive molecules, such as enzymes, enzyme substrates that fluoresce upon cleavage enable detection by separating the quencher from the fluorescence donor in a fluorescence resonance energy transfer (FRET) system. 3 Finally, to recover cells of interest, each compartment must be assessed and potentially sorted based on the intensity of the accumulated secretion signal. To recover cells from microwells, low-throughput liquid handlers, microvalves, optofluidics and micromanipulators are needed. Droplets are typically sorted directly on microfluidic chips using techniques such as dielectrophoresis (DEP), in which spatially varying electric fields generated by electrodes (gray and red structures) exert forces on polarizable droplets. Lab-on-a-particle technologies compatible with commercially available high throughput flow sorters have been demonstrated.