Figure 3.
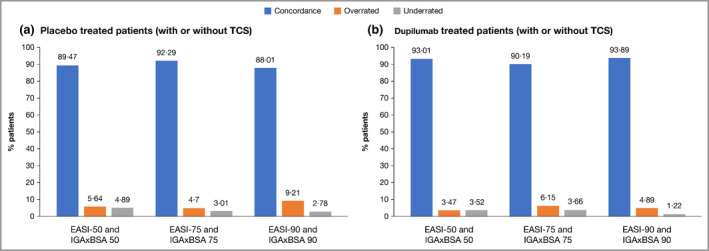
Concordance of IGAxBSA vs. EASI: patients in randomized controlled trials. (a) Placebo‐treated patients, with or without topical corticosteroids (TCS); (b) dupilumab‐treated patients, with or without TCS. BSA, body surface area; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment. Agreement between IGAxBSA and EASI scores was further assessed based on intraclass correlation coefficients and response concordance rates, using improvements from baseline of 50%, 75% and 90% as the response thresholds in both scales. Concordance was calculated as (number of patients without EASI response and without IGAxBSA response) + (number of patients with EASI response and with IGAxBSA response) / the total number of patients with sufficient data for evaluation. Patients were considered overrated if response was achieved based on IGAxBSA, but not achieved based on EASI. Patients were considered underrated if response was achieved based on EASI, but not achieved based on IGAxBSA. Note that the end of treatment visit for SOLO 1&2, CAFÉ, PEDs and ADOL is week 16, end of treatment visit for SOLO‐CONTINUE is week 36, and end of treatment visit for CHRONOS is week 52.
