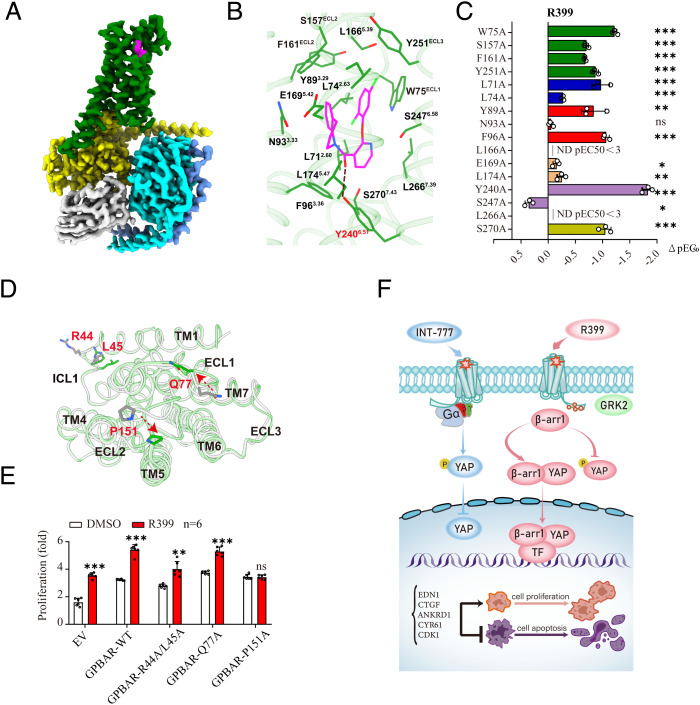
Fig. 8.
Binding of R399 to GPBAR and the structural basis of biased agonism by R399. (A) Cryo-EM density of the GPBAR-Gs-R399 complex. R399 is shown in magenta, GPBAR in green, Gαs in yellow, Gβ in cyan, Gγ in light blue, and Nb35 in gray. (B) Structural view of the insertion of R399 into the ligand pocket. (C) Effects of mutations of the GPBAR ligand binding pocket on the ligand binding of R399. Values are the mean ± SEM of three independent experiments for the wild type (WT) and mutants (P values are as follows: <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, 0.0028, 0.1612, <0.0001, no detectable singnal [ND], 0.0162, 0.0067, <0.0001, 0.0016, ND, and <0.0001 from Top to Bottom for the R399 group). (D) Extracellular view of the GPBAR transmembrane bundle showing the location of the residues with different root mean square deviation between R399 and INT-777–bound GPBAR, colored in green and gray, respectively. Residues with significant conformational changes are marked in red. (E) A549 cells were transfected with empty vector (EV), GPBAR-WT, and GPBAR mutants (R44A/L45A, Q77A, and P151A), respectively. Then the above-mentioned cells were treated with R399 (700 nM) or dimethyl sulfoxide (DMSO) for 72 h (n = 6). (F) Schematic model of molecular mechanism underlying biased GPBAR signaling elicited by INT-777 and R399 through YAP activity in NSCLC cells. GPBAR activation by INT-777 and R399 bidirectionally regulated the YAP pathway through selective activation of Gs and β-arrestin 1 signaling effectors. All data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 based on the Student’s t-test. All results are representative of three independent experiments. ns, not significant.