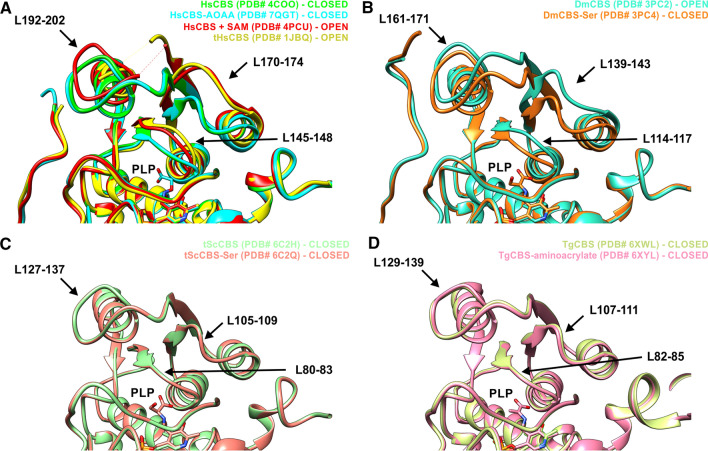
Fig. 7.
Structural comparison of the entrance to the catalytic cavity of CBS enzymes. A Loops L145-148, L170-174 and L192-202 can adopt collapsed (closed) conformation in HsCBS and HsCBS complexed with AOAA. However, the same loops remain relaxed (open) in tHsCBS lacking the regulatory domain or HsCBS in complex with its allosteric activator SAM. B The respective loops in DmCBS are in the open conformation and collapse upon binding of the substrate. C, D) The respective loops in tScCBS and TgCBS show only the closed conformation in both apo enzymes and enzymes with substrates bound to PLP