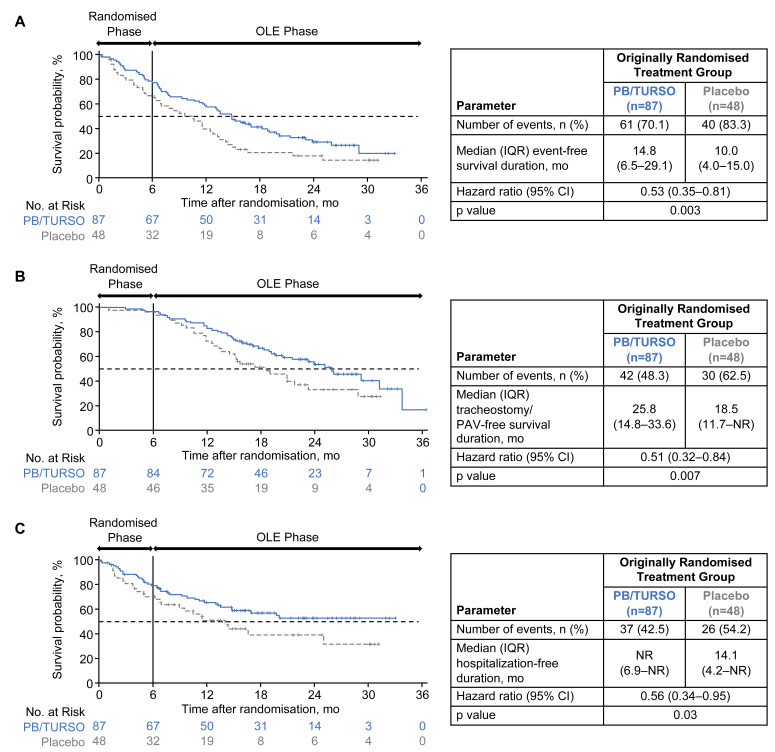
Figure 1.
Kaplan-Meier analyses of time to key events. Time to (A) any key event (ie, death, tracheostomy, PAV or first hospitalisation), (B) death or tracheostomy/PAV and (C) first hospitalisation and corresponding median event-free duration estimates are shown for each originally randomised group in the modified intent-to-treat population (ie, all randomised participants who received at least one dose of originally assigned trial drug and had at least one postbaseline Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised assessment; N=135). HRs and p values were estimated using a Cox proportional hazards model. The numbers at risk exclude participants who experienced the analysed event(s) or were censored before that time point. OLE, open-label extension; PAV, permanent assisted ventilation (defined as non-invasive ventilation >22 hours/day for >7 days); PB/TURSO, sodium phenylbutyrate/taurursodiol.