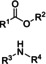
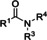
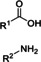
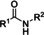
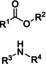
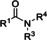
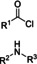
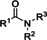
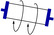
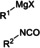
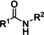
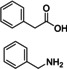
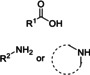
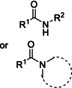
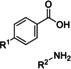
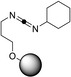
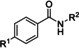
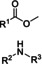
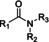
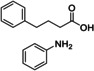
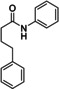
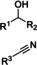
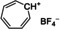
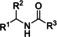
Table 1.
Amide bond formation in segmented continuous flow.
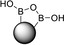
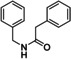
|
Entry |
Starting compound(s) |
Coupling reagent(s)/ catalysts |
Targeted structural scope |
Solvent/ T/p |
Reactor |
# Examples/ yields [%] |
Ref. |
|---|---|---|---|---|---|---|---|
|
1 |
|
TMA |
|
THF 125 °C |
|
17 65–96 |
[66] |
|
2 |
|
– |
|
neat 245–280 °C 25–35 bar |
|
2 93–94 |
[67] |
|
of γ‐Al2O3, MW | |||||||
|
3 |
|
LHMDS |
|
THF 25 °C |
|
25 24–100 |
[68] |
|
4 |
|
TEA |
|
CHCl3 25 °C |
|
28 49–99 |
[69] |
|
5 |
|
CuBr2 (cat.) |
|
THF 25 °C |
|
32 45–98 |
[70] |
|
6 |
|
|
|
toluene 110 °C |
|
1 17[a] |
[71] |
|
7 |
|
CS2, DMAP, Al2O3 |
|
MeCN 200 °C |
|
9 primary+6 secondary 94–98 |
[72] |
|
8 |
|
|
|
DCM–DMF 60 °C |
|
16 76–99[a] |
[73] |
|
9 |
|
ZrO2 |
|
diglyme 160 °C |
|
26 19–99 |
[74] |
|
10 |
|
TiO2/NiFe2O4 |
|
p‐xylene 150 °C |
|
1 0.02 mol gTiO2 −1 s−1 production rate |
[75] |
|
with inductive heating | |||||||
|
11 |
|
|
|
|
|
7 82–93 |
[76] |
|
150 °C |
[a] Only conversions reported.