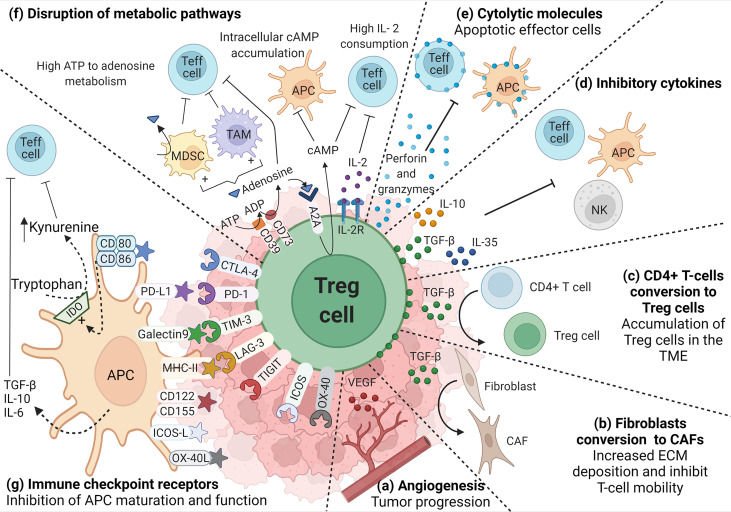
Figure 1.
Role of TI-Treg cells in the TME. TI-Treg cells produce (A) VEGF to promote dysregulated angiogenesis associated with tumor progression and TGF-β that, (B) promote the conversion of fibroblast to cancer-associated fibroblasts (CAFs), and (C) the conversion of CD4+ T-cells to Treg cells promoting their accumulation in the TME. TI-Treg cells also regulate anti-tumor immune responses by producing (D) inhibitory cytokines, such as IL-10, TGF-β and IL-35, inhibiting Teff cells, NKs and APCs, the last two are also inhibited through membrane-bound TGF-β, and (E) cytotoxic molecules such as granzymes and perforin that can directly kill Teff cells and APCs. TI-Treg cells also (F) disrupt Teff cell intracellular metabolism impairing their function by depleting IL-2 in the TME. They express CD39 and CD73 ectonucleotidases that covert ATP and ADP into adenosine, which can engage adenosine receptor A2A on the surface of Teff cells, increasing intracellular cAMP and disrupting their metabolism and function. cAMP also binds to APCs and macrophages inducing tolerogenic myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) that further impact Teff cells. Furthermore, adenosine can also bind A2A in Treg cells promoting the intracellular accumulation of cAMP that can be transferred through gap junctions to Teff cells interfering with their metabolism. Among different molecules that participate in the suppression process, Treg cells highly express (G) immune checkpoint receptors that bind their corresponding ligand in APCs and differently regulate their function (increasing the release of inhibitory cytokines or the upregulation of IDO) promoting an immunosuppressive TME.