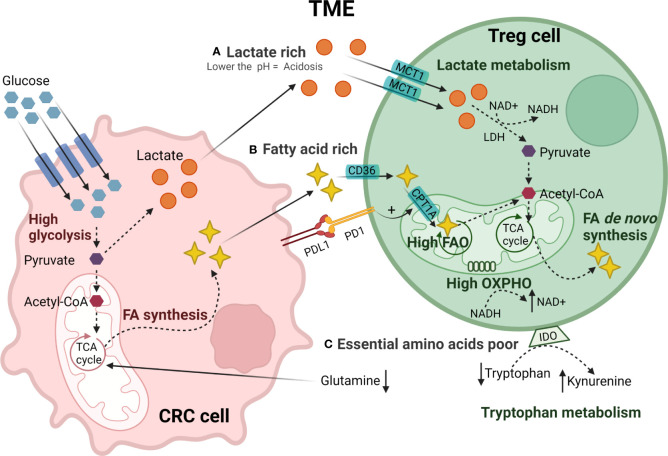
Figure 2.
Metabolic reprogramming of TI-Treg cells in CRC TME. CRC tumor cells have a high glucose uptake generating pyruvate through glycolysis. Pyruvate is converted to acetyl-CoA or lactate that is secreted creating (A) a lactate-rich TME. TI-Treg cells adapt to these metabolic stresses present in the TME. TI-Treg cells can take up lactate through the MCT1 transporter that is subsequently converted to pyruvate by LDH and further to acetyl-CoA. Moreover, CRC tumor cells fuel the tri-carboxylic acid (TCA) cycle in the mitochondria for FA synthesis creating a (B) fatty acid-rich TME. TI-Treg cells can take up FA from the TME through the fatty acid transporter CD36. The CPTR1A transporter drives FA uptake into the mitochondria where it is oxidized by fatty acid oxidation (FAO) to acetyl-CoA. Acetyl-CoA fuels the TCA cycle in the mitochondria for de novo fatty acid synthesis that can be accumulated intracellularly or exported to the TME. The TME has (C) low availability of essential amino acids, particularly glutamine, that is consumed by tumor cells, and tryptophan, that is catabolized to kynurenine by IDO highly expressed by Treg cells.