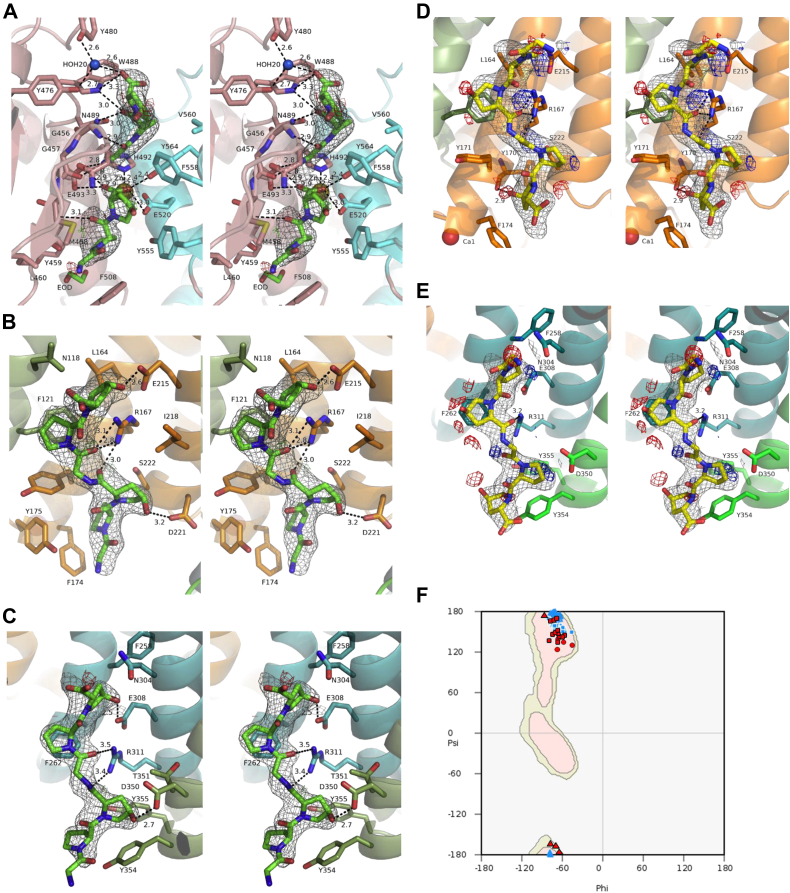
Figure 3.
Structure of Ghcol complexed with Gly-Pro-Hyp in left and right stereo drawings.A–E, close-up views of a, b, and c for Figure 1C are shown in (A–C), respectively. The 2fo–fc omit map (blue) and fo–fc map with model are shown at 1.0 s and ±2.8 s (green and red), respectively. The residues with hydrogen bonds and C–C contacts are labeled. No peaks of fo–fc map appeared in (A–C). D and E, the bound six residues in the activator domain (a and b sites for Fig. 1C) are modeled in a reverse direction and refined. There are several fo–fc map peaks. The omit map is shown in gray color. F, Ramachandran plot. The backbone torsional angles for the collagen triple helix model of (Pro-Pro-Gly)10 (PDB ID: 1K6F) are also plotted (blue). The angles for Gly, Pro, and Hyp are shown with triangles, squares, and circles, respectively. The favored (thin red) and the allowed (thin green) regions are for proline residues generated by COOT. Ghcol, collagenase from Grimontia hollisae strain 1706B; PDB, Protein Data Bank.