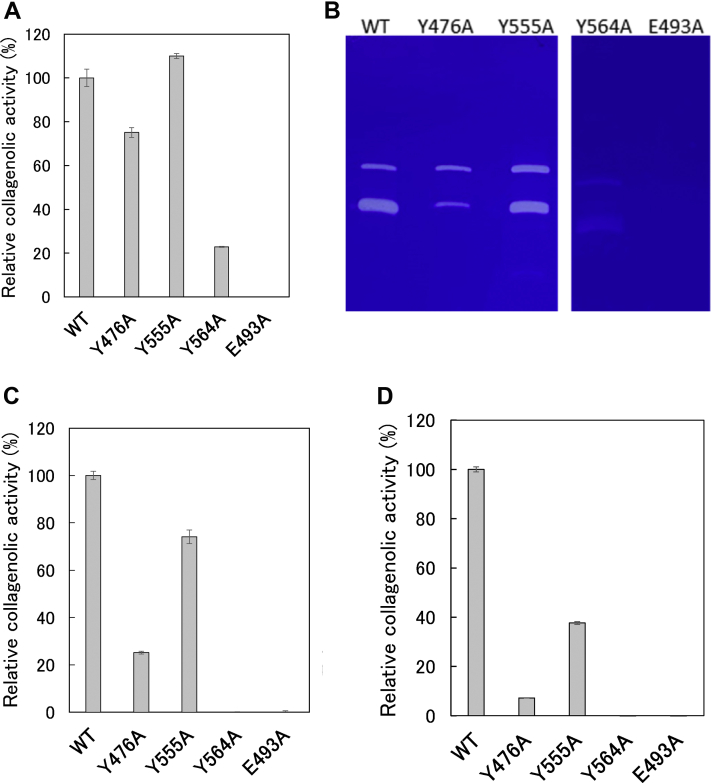
Figure 9.
Activities of Ghcol variants.A, FITC-collagen hydrolytic activities. The reaction was carried out in 50 mM Tris–HCl buffer (pH 7.5), 5 mM CaCl2, 200 mM NaCl, and 1.25 mM acetic acid with 0.50 μg/ml WT, 0.50 μg/ml Y476A, 0.50 μg/ml Y555A, 1.0 μg/ml Y564A, or 100 μg/ml E493A, in the presence of 0.025% FITC-labeled type I collagen at 35 °C. B, gelatin-hydrolyzing activities. About 10 μg/ml WT, 10 μg/ml Y476A, 10 μg/ml Y555A, 100 μg/ml Y564A, or 100 μg/ml E493A were applied to 12.5% polyacrylamide gel containing 0.063% gelatin. Coomassie brilliant blue–stained gels are shown. C, hydrolysis of MOCAc-KPLGL(Dpa)-AR peptide. The reaction was carried out in 5 mM HEPES–NaOH buffer (pH 7.0) with 1.0 μg/ml WT, 3.0 μg/ml Y476A, 1.0 μg/ml Y555A, 100 μg/ml Y564A, or 100 μg/ml E493A, in the presence of 1.0 μM MOCAc-KPLGL(Dpa)-AR at 25 °C. D, hydrolysis of FALGPA. The reaction was carried out in 100 mM Hepes–NaOH buffer (pH 7.5), 200 mM NaCl, 10 mM CaCl2 10 μM ZnCl2 with 1.0 μg/ml WT, 10 μg/ml Y476A, 1.0 μg/ml Y555A, 57 μg/ml Y564A, or 10 μg/ml E493A, in the presence of 80 μM FALGPA at 25 °C. Error bars indicate SD values of triplicate determination. FALGPA, N-[3-(2-furyl)acryloyl]-Leu-Gly-Pro-Ala; MOCAc-KPLGL(Dpa)-AR, (7-Methoxycoumarin-4-yl)acetyl-Lys-Pro-Leu-Gly-Leu-[N3-(2,4-dinitrophenyl)-2,3-diaminopropionyl]-Ala-Arg; Ghcol, collagenase from Grimontia hollisae strain 1706B.