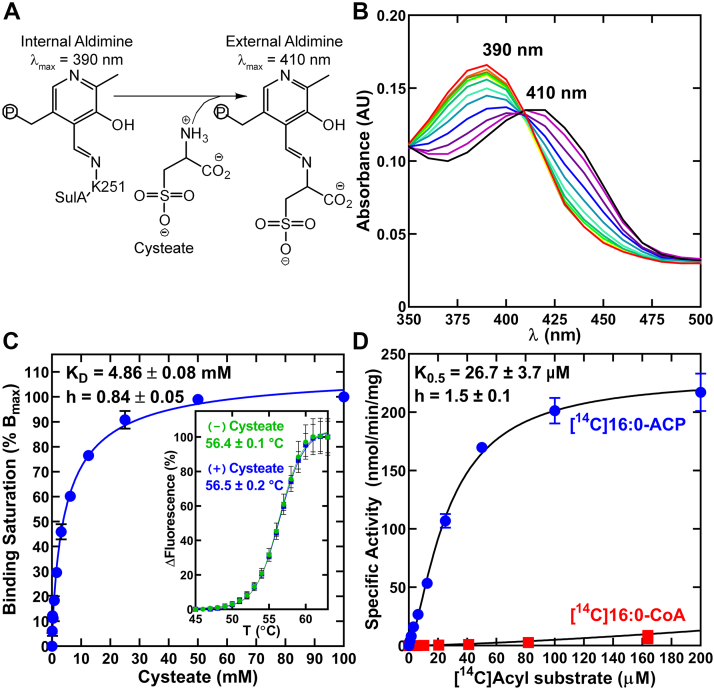
Figure 4.
SulA prefers acyl-ACP as substrate.A, schematic of the cysteate-dependent transition between internal (Amax = 390 nm) and external (Amax = 410 nm) forms of the PLP aldimine. B, cysteate titration (0, 0.097.7, 0.1953, 0.3906, 0.7812, 1.6, 3.1, 6.3, 12.5, 25, 50, and 100 mM; plotted from red to violet) causes a spectral shift in SulA absorption spectrum. C, the data from three independent cysteate titrations were fit to the Hill equation (line) using GraphPad Prism software to calculate the KD and Hill coefficient (h). Inset, protein thermal denaturation analysis shows cysteate binding does not alter the thermal stability of SulA. Points were fit to the Boltzmann equation (n = 3). D, specific activities of SulA calculated as a function of [14C]16:0-ACP (blue) or [14C]16:0-CoA (red). The data are from three independent experiments per acyl substrate, and the enzymatic behavior of SulA was fit to the Hill equation (line). ACP, acyl carrier protein; PLP, pyridoxal phosphate; SulA, cysteate acyl-ACP transferase.