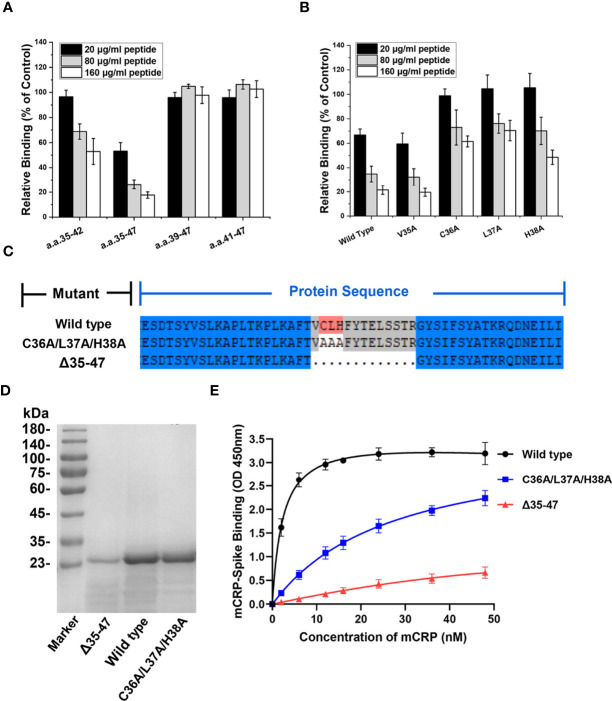
Figure 4.
The N-terminal residues determine the ligand-binding ability of the CBS. (A) CBS mutants with truncations in its N or C terminus were tested for their effects on mCRP binding to the spike RBD (n = 3). The inhibitory effects of CBS were lost following N-terminal truncation but not C-terminal truncation. (B) CBS with point mutations at the indicated N-terminal residues were tested for their effects on mCRP binding to the spike RBD. The three amino acid point mutations at the N-terminus (C36A/L37A/H38A) significantly weakened the ability of the CBS to compete for mCRP and spike RBD binding (n = 3). (C) Schematic showing construction of mCRP 35-47 amino acid deletion mutants (Δ35-47) and C36A/L37A/H38A mutants. (D) The wild type, C36A/L37A/H38A mutant, and Δ35–47 mutants were constructed in E. coli and purified using SEC and SDS-PAGE. (E) mCRP wild type or mutants with altered CBS were expressed in E. coli and purified to test their binding to the spike RBD (n = 3). The binding of mCRP mutants was impaired by mutating the 3 N-terminal residues (C36A/L37A/H38A) or deleting amino acids 35–47. All results are presented as means ± S.E.M.