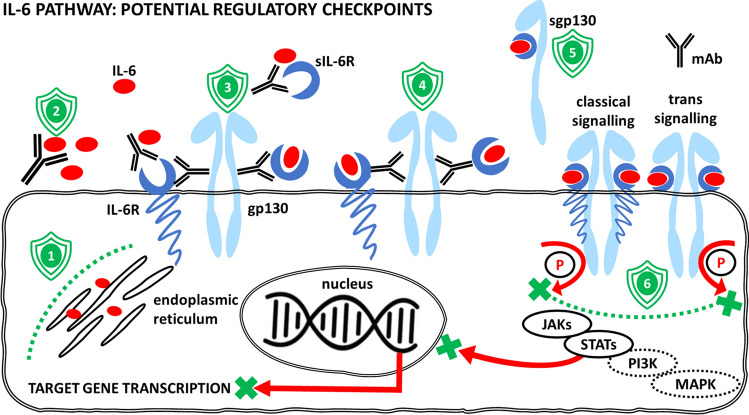
Fig. 5.
Interleukin-6 (IL-6) signalling can be therapeutically regulated at several checkpoints. (1) The production of IL-6 can be diminished (e.g. using curcumin). (2) Once released, the bioavailability of IL-6 can be diminished by neutralizing antibodies (mAb) (e.g. siltuximab). (3) Another therapeutic approach can be based on targeting the interleukin-6 receptor (IL-6R) and/or its soluble form (sIL-6R) (e.g. tocilizumab, sarilumab). This can prevent IL-6R from binding to the cytokine IL-6 and formation of the active cytokine–receptor complex. Alternatively, antibodies or small-molecule inhibitors against the receptor or signal transducer gp130 can prevent the binding of the activated complex (IL-6/IL-6R or IL-6/sIL-6R) to signal transducer glycoprotein gp130 (e.g. bazedoxifene). (4) Antibodies raised against epitopes of signal transducer gp130 can also prevent the binding of the activated complexes (IL-6/IL-6R or IL-6/sIL-6R) (e.g. B-R3). (5) The soluble form of the signal transducer molecule (sgp130) binds the active complex of IL-6/sIL-6R (e.g. olamkicept). This has an inhibitory effect on trans-signalling by reduction of available sIL-6R. It can also help to sequestrate free IL-6. (6) Inhibitors of gp130 kinase activity prevent phosphorylation (P) of downstream signalling molecules (JAKs, STATs, PI3K, MAPK) (e.g. baricitinib), with their consequent translocation to the nucleus, where they regulate target gene transcription. Examples of drugs were selected from Španko et al. (2021)