Abstract
Aim
Primary haemophagocytic lymphohistiocytosis (HLH) is a rare, life‐threatening, hyperinflammatory syndrome generally occurring in early childhood. The monoclonal antibody emapalumab binds and neutralises interferon γ (IFNγ). This study aimed to determine an emapalumab dosing regimen when traditional dose‐finding approaches are not applicable, using pharmacokinetic‐pharmacodynamic analyses to further clarify HLH pathogenesis and confirm IFNγ neutralisation as the relevant therapeutic target in pHLH.
Methods
Initial emapalumab dosing (1 mg/kg) for pHLH patients participating in a pivotal multicentre, open‐label, single‐arm, phase 2/3 study was based on anticipated IFNγ levels and allometrically scaled pharmacokinetic parameters estimated in healthy volunteers. Emapalumab dosing was adjusted based on estimated IFNγ‐mediated clearance and HLH clinical and laboratory criteria. Frequent dosing and emapalumab dose adaptation were used to account for highly variable IFNγ levels and potential target‐mediated drug disposition.
Results
High inter‐ and intra‐individual variability in IFNγ production (assessed by total IFNγ levels, range: 102‐106 pg/mL) was observed in pHLH patients. Administering emapalumab reduced IFNγ activity, resulting in significant improvements in clinical and laboratory parameters and a reduced risk of adverse events, mainly related to pHLH. Modelled outcomes supported dose titration starting from 1 mg/kg, with possible increases to 3, 6 or 10 mg/kg based on re‐evaluation of parameters of disease activity every 3 days.
Conclusions
The variable and unanticipated extremely high IFNγ concentrations in patients with pHLH are reflected in parameters of disease activity. Improved outcomes can be achieved by neutralising IFNγ using frequent emapalumab dosing and dose adaptation guided by clinical and laboratory observations.
Keywords: immunology – inflammation, immunology – monoclonal antibodies, paediatrics – children, pharmacodynamics – modelling and simulation, pharmacodynamics – pharmacokinetics‐pharmacodynamics

What is already known about this subject
Patients with primary haemophagocytic lymphohistiocytosis (HLH), a rare, life‐threatening hyperinflammatory syndrome affecting infants and children, have high circulating levels of interferon γ (IFNγ). The anti‐IFNγ monoclonal antibody emapalumab binds free and receptor‐bound IFNγ, impeding its biological activity. No efficacy/safety data was available from patients when this study was conducted.
What this study adds
IFNγ production can be extremely high and variable over time in primary HLH patients. Its free concentration can be estimated using concentrations of serum CXCL9, produced almost exclusively through IFNγ receptor activation. HLH activity parameters, being affected by IFNγ, can guide emapalumab dose adaptation to achieve IFNγ neutralisation and disease control.
1. INTRODUCTION
Primary haemophagocytic lymphohistiocytosis (HLH) is a rare, life‐threatening syndrome characterised by immune dysregulation and hyperinflammation, primarily affecting infants and young children. 1 Treatment aims to suppress hyperinflammation, allowing patients to undergo allogeneic haematopoietic stem cell transplantation (HSCT), the only curative therapy currently available. 2 , 3 , 4
Treatment has traditionally involved immunochemotherapy with glucocorticoids and etoposide (with or without ciclosporin A), which can be associated with severe myelotoxicity. 4 , 5 Despite continuous attempts to optimise primary HLH treatment, survival has not significantly improved (51% survival probability reported by the HLH‐94 study), 4 , 6 particularly in patients with refractory, recurrent or progressive HLH or intolerance to conventional therapy. 7
IFNγ plays a critical pathogenic role in primary HLH; neutralising IFNγ improved survival and/or reduced HLH signs and symptoms in mouse models 8 , 9 , 10 and high circulating levels of IFNγ are seen in patients with primary HLH. 11 , 12 C‐X‐C motif chemokine ligand 9 (CXCL9) is a chemokine induced almost exclusively by IFNγ in immune cells typically involved in hyperinflammation characteristic of HLH. 13 CXCL9 has been proposed as a biomarker of IFNγ activity and its neutralisation during anti‐IFNγ therapies. 8 , 14 , 15 Assessing the therapeutic potential and pharmacological parameters of a novel anti‐IFNγ treatment is complicated by the rarity of HLH and the severity of the patients' condition requiring a specific methodological approach.
No efficacy or safety data were available from patients with inflammatory disease administered emapalumab, a fully human IgG1 anti‐IFNγ monoclonal antibody binding free and receptor‐bound IFNγ, when this study was conducted. 16 Therefore, in consideration of the severity of HLH, ensuring a neutralising dose of emapalumab from the first administration was of paramount importance in the context of highly variable IFNγ production and an unknown degree of target‐mediated drug disposition (TMDD). This study describes (i) the innovative approach used to determine a dosing regimen for emapalumab in acutely ill infants and children with potentially lethal conditions, wherein a traditional dose‐finding approach was not applicable, and (ii) the contribution of IFNγ‐related pharmacokinetic (PK)‐pharmacodynamic (PD) analyses to the understanding of the pathogenesis of HLH and its treatment.
2. METHODS
Samples for PK and PD analysis were obtained from healthy adult participants enrolled in a randomised, double‐blind, placebo‐controlled, single‐centre, phase 1 study of escalating single intravenous doses of emapalumab (Study NI‐0501‐03, NCT01459562) and from paediatric patients with primary HLH participating in a multicentre, open‐label, single‐arm, phase 2/3 study (Study NI‐0501‐04, NCT01818492 17 ; see study description below) and its long‐term, follow‐up study (Study NI‐0501‐05, NCT02069899). Study NI‐0501‐05 included patients from the NI‐0501‐04 study and patients with HLH who were not eligible to participate in the phase 2/3 study, but had exhausted all possible treatment options and were previously treated with emapalumab on a compassionate use basis. Full details of all studies are provided in Supporting Information Table S1.
All studies were conducted in accordance with the principles set forth in the Declaration of Helsinki, the Guidelines of the International Conference on Harmonisation (ICH) on Good Clinical Practice (GCP) (CPMP/ICH/135/95), European Union Directive 95/46/EC and other applicable regulatory requirements. Independent ethical committee review and approval was obtained prior to initiating the studies and all patients (or their legal guardians) provided written informed consent prior to enrolment.
2.1. Phase 2/3 study design
The phase 2/3 study was initially conceived as a single‐arm phase 2, PK‐ and response‐guided pilot study in patients with primary HLH and disease reactivation following an initial response to conventional therapy. Eligibility criteria were later expanded to include patients with an unsatisfactory response to, or who were intolerant of, conventional therapy, and then to patients who were treatment‐naïve (Supporting Information Figure S1).
PK data from healthy volunteers and nonclinical data were used to calculate the first dose of emapalumab (1 mg/kg every 3 days) based on its potential to neutralise expected circulating IFNγ levels. 18 Subsequent emapalumab dosing could be adjusted based on estimated IFNγ‐mediated clearance assessed using PK parameters, and clinical and laboratory criteria. Peak and trough emapalumab concentrations were used in conjunction with laboratory parameters and clinical data to inform the degree of dose adjustment, preventing suboptimal emapalumab dosing. Emapalumab was administered every 3 days. Frequent emapalumab dosing and possible dose adaptation accounted for the expected variable IFNγ levels and potential TMDD.
The richness of data obtained from the first 19 patients treated with emapalumab during the pilot phase of the study informed the relationship between the PK/PD of emapalumab and its effects on objectively measurable disease parameters, enabling decisions on dose adjustment to be made based on clinical and laboratory parameters only. Accordingly, the set of parameters used to guide dosing was broadened to include additional elements of the HLH‐2004 diagnostic criteria, 19 as described in the protocol amendment that allowed Study NI‐0501‐04 to continue as a pivotal phase 2/3 study (see timeline of amendments in Supporting Information Figure S1).
If a dose increase was deemed necessary during the pivotal phase of the study, emapalumab dosing was selected by the investigator using a predefined algorithm up to 6 mg/kg (or higher, if appropriate; see Supporting Information Table S2). The dose of emapalumab was to be decreased if the clinical and laboratory criteria set for a given dose were no longer applicable. For the entire duration of the study each subsequent dose could only be administered if no specific safety concerns emerged for a given patient and in the presence of a favourable benefit:risk profile, as assessed by the treating physician. Treatment duration was up to 8 weeks, with possible shortening to a minimum of 4 weeks or extension up to HSCT if an appropriate donor was not identified at the end of week 8.
2.2. Bioanalysis
PK samples were analysed for free emapalumab, which was quantified in serum samples using a validated sandwich immunoassay (Gyrolab, Gyros Protein Technologies, Uppsala, Sweden). The lower limits of quantification (LLOQ) were 70 and 62.5 ng/mL in the healthy and HLH populations, respectively.
PD samples were analysed for total IFNγ (circulating free IFNγ + emapalumab‐bound IFNγ), CXCL9, CXCL10 and soluble interleukin receptor 2 (sIL2R) . All PD analyses were performed using a validated electrochemiluminescence immunoassay (Meso Scale Discovery, Meso Scale Diagnostics, LLC, Rockville, MD, USA) with an LLOQ of 50 pg/mL for IFNγ, CXCL10 and sIL2R, and 80 pg/mL for CXCL9 relative to their respective recombinant human reference standards. Prior to being processed, serum samples for IFNγ analysis, along with calibration and quality control samples, were incubated with an excess of emapalumab (50 μg/mL) to push the equilibrium towards the bound form in all samples, avoiding bias due to the dissociation of the emapalumab‐IFNγ complex during the bioanalysis process.
2.3. PK and PK‐PD modelling
Concentration‐time profiles of emapalumab in healthy subjects and patients with HLH (population pharmacokinetic [popPK] models) and CXCL9 in patients with primary HLH (population pharmacokinetic‐pharmacodynamic [popPK‐PD] model) were analysed by nonlinear mixed effects modelling using NONMEM 20 with the first‐order conditional estimation method and interaction. The M3 method for handling of data below the LLOQ 21 was used for CXCL9 because concentrations in 21% of samples were below the LLOQ.
After base model development leading to a two‐compartment model in healthy volunteers, with additional nonlinear clearance in patients, the influence of potential covariates was examined and incorporated in the final popPK models, as described in Supporting Information Table S3. The popPK‐PD model was an indirect response model. The final models were qualified by evaluating the agreement between observed versus predicted concentrations, distribution of individual parameters and distribution of residuals.
The predictive performance of the final models was evaluated using visual predictive checks on observed concentration‐time data. Serum emapalumab concentrations were simulated 500 times for the healthy population and 1000 times for the HLH population using the dose and covariate data from patients used in the PK and PD model development dataset. The 95% confidence intervals for the median and 5th/95th and 2.5th/97.5th percentiles of the simulated PK and PD data, respectively, were then compared visually to the corresponding percentiles of observed data from the phase 2/3 study.
2.4. PD‐efficacy analyses
Relationships between CXCL9 (absolute concentrations or percentage of baseline) and efficacy parameters were explored by graphical analysis for individual disease activity parameters (ferritin, sIL2Rα, D‐dimer, neutrophils, platelets, fibrinogen), and by logistic regression and receiver operating characteristic (ROC) analyses for overall clinical response. 17 Any parameter with a P value <.10 was considered significant. The relative fit for each univariate analysis was assessed using the Akaike information criterion.
2.5. Exposure‐safety analyses
Relationships between emapalumab exposure and safety were analysed by logistic regression for adverse events (AEs; serious AEs, severe AEs, AEs of infections and infusion‐related reactions) and by graphical analysis for selected parameters of renal and liver functions (total bilirubin, alanine aminotransferase and creatinine clearance).
2.6. Software
The popPK and popPK‐PD models were developed using NONMEM Version 7.3.0 software (ICON plc, Dublin, Ireland). Additional analyses were performed using R software version 3.2.3 and/or SAS software version 9.4.
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/2022. 22 , 23
3. RESULTS
3.1. Clinical pharmacology framework
A PK‐PD‐efficacy/safety framework was used to guide the clinical development of emapalumab, based on an anticipated semimechanistic model (Figure 1). Patients with HLH were expected to have an activated IFNγ pathway. Emapalumab was expected to bind free IFNγ to form an IFNγ‐emapalumab complex. If free emapalumab was in excess, and assuming concentration‐independent clearance of the IFNγ‐emapalumab complex, the total IFNγ concentration at equilibrium would be considered proportional to IFNγ production, as the amount of receptor‐bound IFNγ can be considered negligible, given the circulating total IFNγ abundance. Hence, changes in total IFNγ concentration indicate proportional changes in IFNγ production.
FIGURE 1.
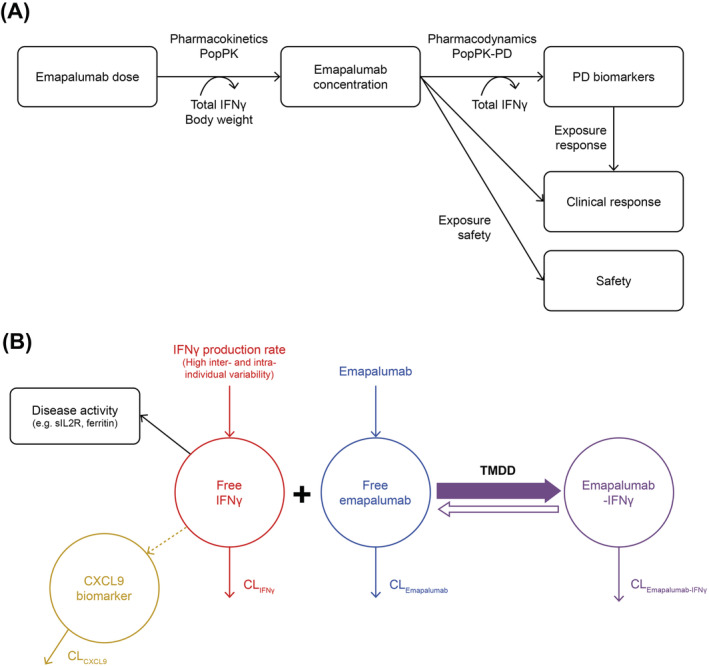
Schematic representation of (A) the overall PK‐PD‐efficacy/safety analysis strategy for emapalumab in patients with HLH and (B) PK‐PD‐disease interactions between IFNγ, emapalumab, CXCL9 and HLH disease activity. Note: In patients with HLH, the endogenous production of IFNγ (red) is exacerbated and leads to high circulating concentrations of free IFNγ. After administration, emapalumab (blue) binds reversibly to IFNγ, reducing its free concentration, and leads to the formation of an IFNγ‐emapalumab complex (violet), which is in equilibrium with the free moieties. To confirm the neutralisation of IFNγ by emapalumab, the decrease in serum concentration of CXCL9 (orange), a chemokine almost exclusively produced through the activation of the IFNγ receptor, is monitored. As such, CXCL9 can be considered as a biomarker of free IFNγ activity, hence the pharmacological activity of emapalumab in neutralising IFNγ. The decrease in free IFNγ is expected to improve disease status, which can be monitored by parameters such as sIL2R and ferritin (black). These parameters are included in the panel of HLH diagnostic criteria and considered as components of response to HLH treatments. All components described above (ie, free IFNγ, free emapalumab, IFNγ‐emapalumab complex, CXCL9, sIL2R and ferritin) have their own intrinsic clearance and their concentrations correspond to an equilibrium between production and elimination. Furthermore, for emapalumab, the formation of the complex with IFNγ corresponds to an additional clearance proportional to the IFNγ production. This phenomenon is known as TMDD. In patients with HLH receiving emapalumab, serum concentrations of free emapalumab (before drug administration), total IFNγ (ie, free IFNγ + IFNγ‐emapalumab complex) and CXCL9 have been measured. When free emapalumab is in excess, total IFNγ concentration at equilibrium can be considered as proportional to the IFNγ production and changes in total IFNγ concentration indicate proportional changes in IFNγ production. CL, clearance; CXCL9, chemokine (C‐X‐C motif) ligand 9; IFNγ, interferon γ; HLH, haemophagocytic lymphohistiocytosis; PD, pharmacodynamic; PK, pharmacokinetic; Pop, population; sIL2R, soluble interleukin‐2 receptor; TMDD, target mediated drug disposition
Furthermore, emapalumab would be expected to reduce IFNγ‐mediated biochemical activity, which could be monitored by measuring serum concentrations of CXCL9. 24 , 25 Accordingly, reduced IFNγ activity would also be expected to translate into observable improvements in the clinical and laboratory parameters of disease activity. 17 , 19
However, all components of the model in Figure 1B (ie, free IFNγ, free emapalumab, IFNγ‐emapalumab complex and CXCL9) have their own intrinsic clearance and their concentrations correspond to an equilibrium between production and elimination. Of note, the formation of the IFNγ‐emapalumab complex results in an increased rate of emapalumab clearance that is proportional to IFNγ production.
3.2. PK model development in healthy subjects
No evidence of nonlinear elimination was observed in exploratory graphical analyses of samples collected for PK analysis from 14 healthy subjects given emapalumab, particularly once dosing was normalised to a 1 mg/kg dose (see Supporting Information Table S4A and Figure S2 ). Therefore, a basic two‐compartment model, parameterised in terms of clearance (total clearance [CL] and inter‐compartmental clearance [Q]), volumes of distribution (central volume of distribution [V1] and peripheral volume of distribution [V2]), was developed with residual variability being described using an additional log‐scale term (Supporting Information Table S5 ). The volume of distribution and clearance of emapalumab were low (5.85 L and 0.171 L/day, respectively) and its half‐life long (25 days). These findings are consistent with the expected PK profile of a monoclonal antibody. Covariate analysis indicated that V1 declined (5.20 → 2.65 L) with increasing emapalumab dose (0.75 → 225 mg), suggesting a possible TMDD effect due to emapalumab binding IFNγ.
Median total IFNγ concentrations increased from below the limit of detection (<50 pg/mL) up to maximums of 549 and 530 pg/mL after administration of emapalumab 1 and 3 mg/kg, respectively. Steady‐state total IFNγ concentrations were generally obtained within 1 week of administering emapalumab and maintained for more than 8 weeks.
3.3. Calculation of the first dose in patients with primary HLH
The first dose of emapalumab to be administered to patients with HLH was calculated based on parameters defined in Supporting Information Table S6. Simulations performed using anticipative models developed using in vitro data and in vivo data from healthy volunteers estimated that an emapalumab dose of 1 mg/kg every 3 days would neutralise 99% of IFNγ in patients with HLH and baseline IFNγ concentrations up to 1700‐3400 pg/mL (Supporting Information Figure S3 ). Furthermore, the simulations confirmed that a dosing interval of 3 days would be necessary to counteract the TMDD effect in patients with high IFNγ production. However, due to the degree of uncertainty inherent in the modelled predictions, subsequent dose increases for individual patients were anticipated once data on measured emapalumab concentrations and observed clinical response were available. Therefore, an emapalumab concentration and laboratory/clinical parameter control was implemented in the protocol of the pilot phase 2 study.
3.4. PopPK model development in patients with HLH
Starting from the two‐compartment PK model developed in healthy subjects, individual concentration‐time profiles in patients with HLH (see Supporting Information Table S4B for baseline characteristics) were analysed to develop a popPK model that included two CL components: slow linear CL and a target‐mediated CL dependent on IFNγ production (CLNL) (Table 1). In addition, total IFNγ concentration (free + bound), an indicator of IFNγ production, was incorporated into the model as a time‐varying covariate for CLNL.
TABLE 1.
PK parameters of the final popPK model developed using data from patients with primary HLH participating in the NI‐0501‐04/‐05 studies a
| Parameter | Description | Estimate (95% CI) | RSE, % |
|---|---|---|---|
| CLL, L/h/70 kg | Linear clearance | 0.0116 (0.00486‐0.0183) | 29.7 |
| CLNL, L/h/70 kg | Nonlinear clearance (IFNγ‐dependent) | 0.133 (0.0456‐0.220) | 33.5 |
| V1, L/70 kg | Central volume of distribution | 4.16 (3.80‐4.52) | 4.4 |
| Q, L/h/70 kg | Intercompartmental clearance | 0.102 (0.0495‐0.155) | 26.3 |
| V2, L/70 kg | Peripheral volume of distribution | 5.55 (3.30‐7.80) | 20.7 |
| CLNL_IFNγ | Influence of IFNγ on CLNL (power function) | +0.746 (0.567‐0.925) | 12.3 |
| CL_BWV_BW | Allometric exponent of body weight on CLs | +0.886 (0.680‐1.09) | 11.9 |
| Allometric exponent of body weight on vs | 1 fixed | … | |
| IIV | Estimate (IIV %) | Shrinkage, % | |
| OMEGA2_CLI | Variance of CL | 0.240 (49.0) | 4.1 |
| OMEGA2_V1 | Variance of V1 | 0.0721 (26.9) | 7.1 |
| OMEGA2_V2 | Variance of V2 | 0.766 (87.5) | 5.4 |
| OMEGA2_V1_V2 | Correlation (V1/V2) | +0.728 (−) | … |
| RSV | Estimate (RSV %) | Shrinkage, % | |
| SIGMA | SD of residual (additive in log domain) | 0.306 (30.6) | 2.4 |
Note: CL = (CLL + CLNL × (total IFNγ/1,000,000)^CLNL_IFNγ) × (BW/70)^CL_BW with total IFNγ in pg/mL and BW in kg.
Abbreviations: BW, body weight; CI, confidence interval; CL, clearance; CLL, linear clearance; CLNL, nonlinear clearance; HLH, hemophagocytic lymphohistiocytosis; IFNγ, interferon‐γ; IIV, interindividual variability; RSE, relative standard error; RSV, residual variability; SD, standard deviation; V, volume of distribution.
Model developed using 2414 measurements of emapalumab concentrations from the 33 patients with primary HLH treated in the NI‐0501‐04 study and 16 HLH patients who received emapalumab in compassionate use. Objective function value = −2851.507.
High inter‐ and intra‐individual variability in IFNγ production (ranging from 102 to 106 pg/mL) was observed in patients with HLH treated with emapalumab (Figure 2) and higher total IFNγ concentrations were found to result in greater CLNL for patients with a total IFNγ concentration >104 pg/mL (Figure 3). When total IFNγ was in the range of 103‐106 pg/mL, total emapalumab CL (linear + target‐mediated) ranged from 0.0124 to 0.145 L/h/70 kg, with corresponding terminal half‐lives from 23.6 to 3.09 days. No other selected covariates (age, sex, ethnicity, renal or liver parameters) had a statistically significant effect on emapalumab PK.
FIGURE 2.
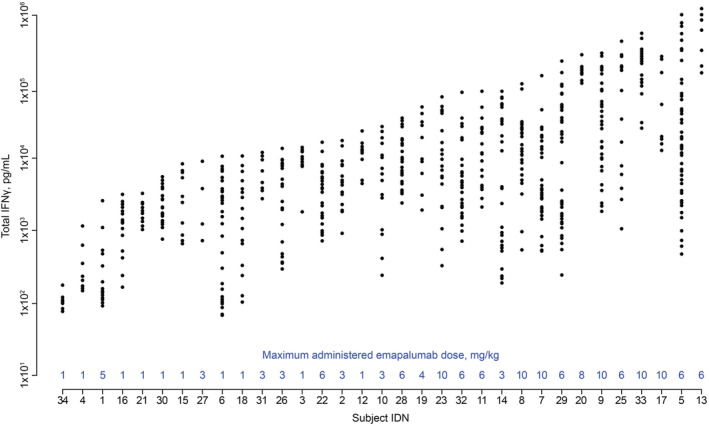
Total IFNγ concentrations in patients with HLH up to the last dose of emapalumab or HSCT in studies NI‐0501‐04/‐05. Note: The maximum administered dose of emapalumab is given in blue above the x axis. Concentrations before day 3 were omitted as steady state was not yet reached. IDN numbers are arbitrary designations to allow comparisons between figures. Patient 1 was erroneously administered one dose of emapalumab 5 mg/kg, but otherwise received 1 mg/kg during the entire treatment period. HLH, haemophagocytic lymphohistiocytosis; HSCT, haematopoietic stem cell transplant; IFNγ, interferon γ; IDN, identifier
FIGURE 3.
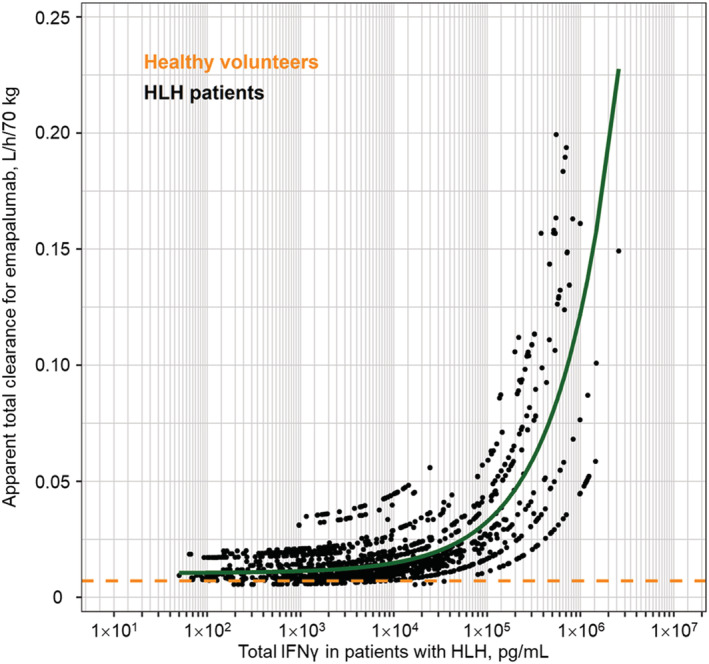
Estimated apparent total clearance of emapalumab versus total IFNγ concentrations in patients with primary HLH participating in studies NI‐0501‐04/‐05 or having received emapalumab in compassionate use. Note: The dots are individual predicted clearances in patients. The green line is the population predicted clearance. The orange dotted line is the clearance in healthy subjects. HLH, haemophagocytic lymphohistiocytosis; IFNγ, interferon γ
Goodness‐of‐fit plots (Supporting Information Figure S4) and visual predictive checks (Supporting Information Figure S5) indicated that the popPK model was satisfactory for simulating emapalumab concentrations over time and by total IFNγ concentrations in patients with HLH.
3.5. PopPK‐PD model development in patients with primary HLH
To quantify emapalumab‐mediated neutralisation of IFNγ, the relationship between emapalumab and CXCL9 concentrations over time was modelled using an indirect‐response model (Supporting Information Figure S6). Administering emapalumab was found to reduce IFNγ activity, as demonstrated by the significant decrease in post‐emapalumab CXCL9 concentrations versus baseline (Figure 4 and Supporting Information Figure S7), while total IFNγ concentration was identified as a significant explanatory variable for CXCL9 concentration (Supporting Information Table S7).
FIGURE 4.
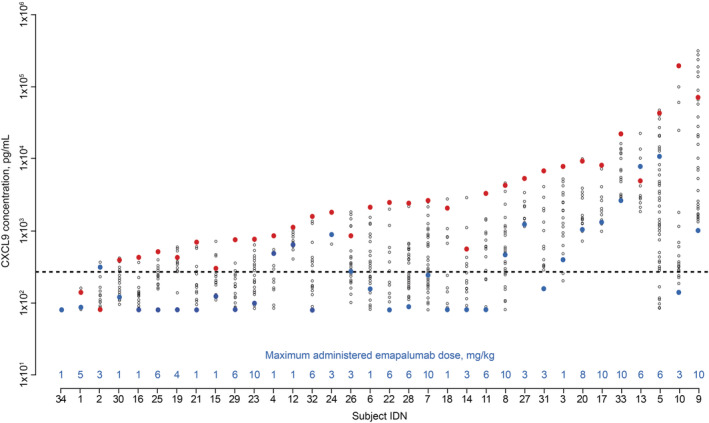
Total CXCL9 concentrations in patients with primary HLH up to the last dose of emapalumab or HSCT in patients with primary HLH participating in the NI‐0501‐04 study. Note: Red dots indicate CXCL9 levels at study entry. Blue dots indicate levels at end of treatment of study NI‐0501‐04. Black dots indicate values in between study entry and last emapalumab dose. The horizontal dotted line indicates the 95th percentile of CXCL9 levels in healthy subjects. IDN numbers are arbitrary designations to allow comparisons between figures. CXCL9, chemokine (C‐X‐C motif) ligand 9; HLH, haemophagocytic lymphohistiocytosis; HSCT, haematopoietic stem cell transplant; IDN, identifier
The model parameters indicated that the 50% inhibitory concentration (IC50) of emapalumab (approximately 25 ng/mL) was lower than the circulating concentrations of emapalumab after multiple administrations of 1 mg/kg doses (median trough >10 000 ng/mL), indicating strong neutralisation (>99%) was required to achieve a PD effect because of high circulating total IFNγ levels. Furthermore, the equilibrium half‐life of CXCL9 versus the changes in emapalumab or IFNγ levels was approximately 1.2 days, indicating a slight delay in the measured CXCL9 response.
Covariate analysis indicated that body weight, age and sex did not have significant effects on the emapalumab‐IFNγ‐CXCL9 relationship. Dexamethasone dose was identified as a possible covariate (Supporting Information Table S7). Increasing dexamethasone dose tended to result in reduced CXCL9 concentrations, although the effect became much less pronounced when dexamethasone was co‐administered with emapalumab (Supporting Information Figure S8). The model equations for the full model are provided in the footnote of Supporting Information Table S7.
Goodness‐of‐fit plots (Supporting Information Figure S9) and visual predictive checks (Supporting Information Figure S10) indicated that the popPK‐PD model was satisfactory for simulating CXCL9 concentrations in the presence of time‐varying emapalumab and total IFNγ concentrations. Examples of individual observed versus predicted measurements are presented in Supporting Information Figure S11. The model was also considered appropriate for use across a wide range of observed emapalumab and total IFNγ concentrations.
3.6. Exposure‐efficacy analysis in patients with primary HLH
Emapalumab‐induced decreases in CXCL9 were associated with significant improvements in clinical and laboratory parameters of HLH in most patients, which were documented within 6 days of first administering emapalumab (Figure 5). ROC analysis also indicated that decreases in CXCL9 were predictive of overall response at CXCL9 threshold concentrations of 787 pg/mL at week 2 and 292 pg/mL at the end of treatment (Supporting Information Table S8).
FIGURE 5.
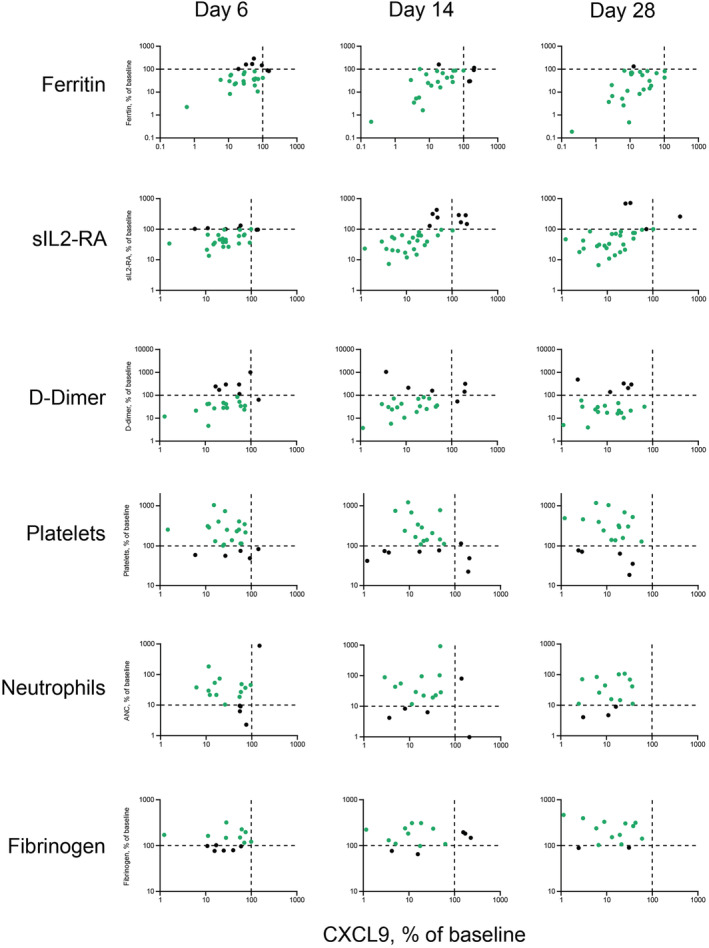
Log‐scale comparison of changes in baseline CXCL9 and laboratory parameters in patients with primary HLH administered emapalumab in studies NI‐0501‐04/‐05 who had abnormal ferritin levels, sIL2Rα levels, D‐dimer levels, platelet counts, neutrophil counts or fibrinogen levels at baseline. Note: Values are percent of baseline. Green dots indicate improvement in a parameter. Black dots indicate no change or worsening in CXCL9 or laboratory parameter. CXCL9, chemokine (C‐X‐C motif) ligand 9; HLH, haemophagocytic lymphohistiocytosis; SD, study day; sIL2‐RA, soluble interleukin‐2 receptor α
While CXCL9, CXCL10 and total IFNγ were found to be significant (P < .10) prognostic factors for overall response at the end of treatment (Supporting Information Table S9), CXCL9 appeared to be the strongest factor, followed by total IFNγ (ie, in the presence of emapalumab) and then CXCL10. Multivariate logistic regression analysis of all prognostic factors identified in the univariate analysis followed by a stepwise backward deletion did not identify any multivariate effects.
3.7. Exposure‐safety analysis in patients with primary HLH
Changes in total bilirubin, alanine aminotransferase and creatinine clearance were not associated with emapalumab exposure, a finding consistent with logistic exposure‐safety regression analyses indicating that the incidence of AEs, such as infection, the vast majority of which were assessed as being HLH‐ rather than emapalumab‐related, did not increase as a function of increasing emapalumab concentrations (Figure 6). On the contrary, the incidence of AEs decreased with higher emapalumab concentrations (Supporting Information Table S10).
FIGURE 6.
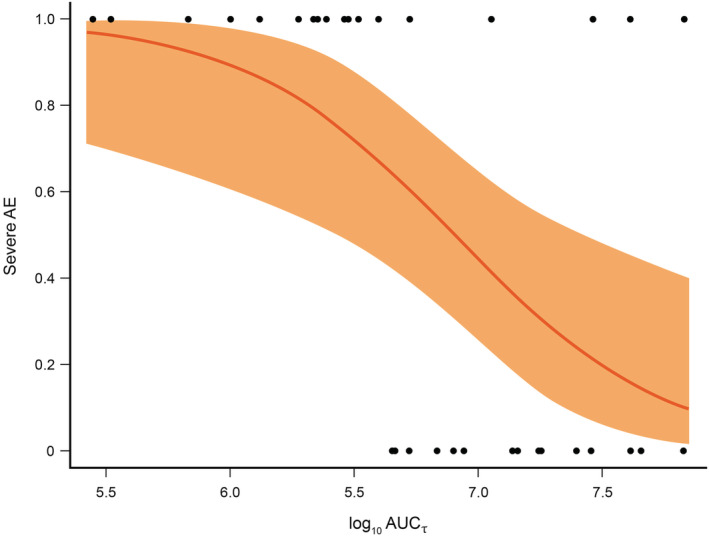
Predicted risk of severe AEs as a function of the log10‐transformed AUCτ of emapalumab. Note: The orange area represents the 95% CIs. The black dots represent the actual observed severe AEs throughout treatment with emapalumab (0 = no severe AE and 1 = severe AE) with their corresponding AUCτ. AE, adverse event; AUCτ, area under the curve to over a dosing interval; CI, confidence interval
3.8. PK/PD/efficacy simulations in patients with primary HLH
To support the proposed dosing of emapalumab in patients with primary HLH, the popPK and popPK‐PD models were combined, and simulated CXCL9 concentration‐time profiles compared with the levels of CXCL9 associated with overall clinical response at week 2 and end of treatment from the ROC analysis, as well as with the likelihood of overall response from the logistic regression analysis (Supporting Information Figure S12). Patients with low total IFNγ were expected to achieve CXCL9 levels associated with a positive overall response with 1 mg/kg dosing every 3 days, which was consistent with the proportion (approximately one‐third) of patients with HLH administered this dose achieving an overall response. Most of these patients had low‐to‐moderate total IFNγ concentrations.
However, the simulations also indicated that patients with high total IFNγ concentrations would require higher doses of emapalumab to lower CXCL9 levels below the thresholds associated with overall response, which was also consistent with observed outcomes wherein patients who received higher doses of emapalumab – because of an unsatisfactory response – tended to have higher total IFNγ concentrations at study entry (Figure 2). Accordingly, these simulations supported proposed dose titration starting from 1 mg/kg, with possible increases to 3, 6 or up to 10 mg/kg based on re‐evaluation of clinical response every 3 days during the pivotal phase 2/3 study.
4. DISCUSSION
Detailed insight into the intra‐ and interindividual variability of IFNγ production assessed by total IFNγ indicated that the variation and instability observed in the clinical presentation of patients with primary HLH reflects IFNγ levels, which were up to 1000 times higher than those observed in healthy subjects. Of note, circulating IFNγ may represent only a fraction of the IFNγ produced since a high proportion potentially remains in inflamed tissues, only becoming measurable following administration of an anti‐IFNγ antibody (ie, emapalumab), which alters the balance of the tissue‐circulation equilibrium towards higher IFNγ in circulation.
Biologically active free IFNγ is no longer measurable following emapalumab administration. This study shows through modelling that CXCL9 is a reliable marker of the IFNγ pathway activation, further corroborated by the observed correlation of CXCL9 with changes in disease activity parameters in patients with primary HLH, as previously described in other settings. 8 , 14 , 15
These additional insights into the pathophysiology of HLH were key to informing the dosing regimen for emapalumab during the pivotal study demonstrating its efficacy and safety in patients with primary HLH. 17 In particular, an individualised and dynamic approach to neutralising this variable, and potentially extremely high, production of IFNγ in patients with HLH was required, especially as the degree of IFNγ neutralisation was correlated with changes in disease parameters.
As expected, given the genetic basis of primary HLH, emapalumab did not alter IFNγ production, but neutralised its activity. A 1 mg/kg dose was adequate in patients with low‐to‐moderate IFNγ levels at study entry, while dose increases up to 10 mg/kg were generally required to neutralise IFNγ in patients with higher IFNγ levels. Importantly, reducing CXCL9 levels below a threshold of approximately 300 pg/mL was associated with overall response at the end of treatment, regardless of IFNγ levels at study entry. Although CXCL9 may be a clinically useful biomarker, it was determined that disease activity parameters observed in individual patients given emapalumab 17 were an appropriate method for guiding dose adaptation by the treating physician because these parameters generally reflected the PD effect of emapalumab.
Background immunosuppressant therapy with dexamethasone alone was not sufficient to overcome high IFNγ production in patients with primary HLH. In fact, the relative reduction in IFNγ activity attributed to dexamethasone when co‐administered with emapalumab was low, which is consistent with the targeted mode of action of emapalumab being expected to be more effective at neutralising IFNγ activity than glucocorticoids. In addition, any dexamethasone‐related decrease in CXCL9 levels was less pronounced when high IFNγ inhibition had already been achieved with emapalumab, indicating that dexamethasone doses could be lowered, as foreseen in the study protocol, for patients with HLH administered emapalumab without negatively impacting IFNγ neutralisation.
Of note, emapalumab is the first drug being specifically developed for the treatment of HLH. Therefore, in contrast with therapies currently recommended for the first‐line management of primary HLH, such as etoposide and ciclosporin A (used off‐label), emapalumab is the only drug for the treatment of primary HLH for which PK or PD data have been generated to support its recommended dosing regimen.
This study also confirmed that the PK properties of emapalumab are consistent with those expected of a monoclonal antibody, namely a low volume of distribution and slow clearance. These characteristics are consistent with observations of another anti‐IFNγ monoclonal antibody, AMG‐811. 26 Interestingly, the influence of TMDD on AMG‐811 clearance has not been reported, possibly because this could not be accurately quantified in patients with systemic lupus erythematosus, nor could TMDD for emapalumab be quantified in PK studies in healthy subjects due to low or undetectable IFNγ levels. However, marked TMDD was anticipated when calculating the first emapalumab dose to be administered to patients with primary HLH with an additional nonlinear elimination path through the binding of emapalumab to IFNγ incorporated using a Michaelis‐Menten equation. The anticipated TMDD of emapalumab, and its magnitude, were subsequently confirmed by monitoring PK parameters after administering each dose of emapalumab in individual patients and by a post hoc popPK analysis.
Data from this study did not allow full incorporation of the TMDD process into models because dose adaptation and a short dosing interval (every 3 days) may have saturated the extra elimination pathway and circumvented observation of the nonlinear elimination phase. Parameters of the quasi‐steady‐state or Michaelis‐Menten approximations were not identifiable based on the emapalumab concentrations alone. To help, concentrations of the drug‐target complex over time could have been used. However, at an individual patient level, changes in the target production were highly variable (up and down) at unpredictable times. Thus, total IFNγ could not be modelled as such and used in a TMDD model. Therefore, the extra elimination path was modelled using NLCL, the magnitude of which was directly related to IFNγ production assessed by time‐varying total IFNγ concentration. At a total IFNγ concentration >10 000 pg/mL, target‐mediated clearance became significant compared with linear emapalumab clearance, justifying dose adaptation to achieve strong IFNγ neutralisation.
This study had a few limitations: the small sample size, the requirement for allometric scaling to predict the PK characteristics of emapalumab across a wide range of body weights as primary HLH affects a paediatric population and the fact that a semimechanistic TMDD model could not be implemented due to the adaptive nature of the study design dictated by the life‐threatening nature of HLH.
In conclusion, primary HLH is characterised by a variable clinical presentation and evolution that is associated with intra‐ and interindividual variable IFNγ production that can reach extremely high levels. During the development of emapalumab, the first targeted treatment for HLH, an innovative approach based on frequent administration, and dose adaptation based on PK and disease parameters, was applied to identify a safe and efficacious dosing strategy in this fragile population of infants and young children with a rapidly evolving life‐threatening disease. The data generated and analyses performed allowed the relationship between the PK and PD properties of emapalumab to be described at population and individual patient levels, confirming the pathogenic role of IFNγ in primary HLH, as demonstrated by the relationship between improvements in disease activity and IFNγ neutralisation. Furthermore, CXCL9 levels represent a reliable tool for estimating IFNγ production and activity. Finally, neutralising IFNγ with the anti‐IFNγ antibody emapalumab offers a personalised therapeutic option for managing a disease characterised by a dismal prognosis.
CONTRIBUTORS
M.B.J., F.L., M.B. and C.dM. were involved in designing the study, writing the study protocol, performing the clinical part of the study and collecting the data. P.J., C.L., E.S., M.B. and C.dM. were involved in the (statistical) analysis of the data. All authors participated in the drafting of the manuscript and approved the final version prior to submission.
CONFLICT OF INTEREST
P.J., C.L., E.S., M.B.J., M.B. and C.dM. are paid consultants of Sobi. M.B.J. has participated in scientific advisory board meetings for Sobi. F.L. has acted as an unpaid consultant to Sobi, reviewing efficacy and safety data from clinical studies. M.B. and C.dM. are former employees of Sobi.
5.
OPEN RESEARCH BADGES
This article has earned a Preregistered Research Designs badge for having a preregistered research design, available at https://clinicaltrials.gov/ct2/show/NCT01459562; https://clinicaltrials.gov/ct2/show/NCT01818492; https://clinicaltrials.gov/ct2/show/NCT02069899.
Supporting information
Supporting Information Table S1Study designs
Supporting Information Table S2 Clinical and laboratory criteria to guide dose increases in the pivotal phase 2/3 study (Study NI‐0501‐04) [1]
Supporting Information Table S3 Covariate analysis and selection in PK models
Supporting Information Table S4 Baseline characteristics of healthy subjects (phase 1 NI‐0501‐03 study; A) and patients with HLH (phase 2/3 NI‐0501‐4/NI‐0501‐05 studies and patients with compassionate use experience; B)a
Supporting Information Table S5 Parameter estimates for the final popPK model in healthy subjects
Supporting Information Table S6 Parameters applied to data from healthy subjects administered emapalumab in a phase 1 study (Study NI‐0501‐03) when calculating the first dose of emapalumab in the pilot phase 2 study (Study NI‐0501‐04) in patients with primary HLH
Supporting Information Table S7 Comparison of model parameters of the base model (RUN506b) and the same model with covariate effect of corticosteroid doses (RUNDEXGAM) using data from patients with HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate use
Supporting Information Table S8 Summary statistics of the ROC analyses for the CXCL9 biomarkers resulting in a significant discrimination between patients with HLH with and without a clinical response using data from patients administered emapalumab in studies NI‐0501‐04/−05
Supporting Information Table S9 Summary statistics of the univariate logistic regression analysis of the clinical response at the end of treatment among patients with HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate use
Supporting Information Table S10 Summary statistics of the univariate logistic regression analysis of the AEs related to emapalumab exposure parameters among patients with HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate use
Supporting Information Figure S1 NI‐0501‐04 and NI‐0501‐05 original study schemas and subsequent amendments
Supporting Information Figure S2 Exploratory graphical analysis of plasma emapalumab concentrations on a semi‐log scale for individual healthy subjects over time (Study NI‐0501‐03)
Supporting Information Figure S3 Predictions of emapalumab concentration‐time profiles after the first administration of 1 mg/kg in patients with HLH of 23 kg with various levels of Vmax a
Supporting Information Figure S4 Goodness‐of‐fit plots presenting (A) observations versus population predictions and (B) observations versus individual predictions using the final popPK model for patients with primary HLHa
Supporting Information Figure S5 Visual predictive check of simulated versus observed plasma emapalumab concentrations over time (A) and by total IFNγ concentration (B)a
Supporting Information Figure S6 Inhibition by emapalumab of the IFNγ‐induced CXCL9 productiona
Supporting Information Figure S7 CXCL9 concentrations as a function of time after the first emapalumab administration in patients with primary HLH participating in the pilot phase 2 study (Study NI‐0501‐04; N = 50)
Supporting Information Figure S8 Effect of glucocorticoid doses on CXCL9 steady‐state concentrations at increasing emapalumab concentrations based on data derived from patients with primary HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate use
Supporting Information Figure S9 Goodness‐of‐fit plots of CXCL9 derived from popPK‐PD analyses from patients with HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate usea
Supporting Information Figure S10 Visual predictive checks of RUN506b. Simulated CXCL9 concentrations as a function of emapalumab concentrations (left graph)a and total IFNγ concentration (right graph) versus observed values from patients with HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate use
Supporting Information Figure S11 Examples of concentration‐time profiles of free emapalumab (blue, left log axis), total IFNγ (red, left log axis), model derived free IFNγ (black, left log axis) and CXCL9 (green, right log axis) for individual patientsa
Supporting Information Figure S12 Simulated CXCL9 concentration‐time profiles for individual patients with HLH and differing bodyweight or total IFNγ levels administered emapalumab 1‐10 mg/kg
ACKNOWLEDGEMENTS
The authors would like to thank Geneviève Lapeyre, formerly of NovImmune, for her contribution to the protocol development and safety data analysis and the Bioanalysis team at NovImmune/Swedish Orphan Biovitrum AB (publ) (Sobi). Funding for this study was provided by Sobi, and by a grant from the European Commission through the First Targeted Therapy to Fight Hemophagocytic Lymphohistiocytosis (FIGHT HLH) program. Medical writing support was provided by Blair Hesp PhD CMPP of Kainic Medical Communications Ltd (Dunedin, New Zealand), which was funded by Sobi.
Jacqmin P, Laveille C, Snoeck E, et al. Emapalumab in primary haemophagocytic lymphohistiocytosis and the pathogenic role of interferon gamma: A pharmacometric model‐based approach. Br J Clin Pharmacol. 2022;88(5):2128-2139. doi: 10.1111/bcp.15133
FUNDING INFORMATION
Funding for this study was provided by Sobi, and by a grant from the European Commission through the First Targeted Therapy to Fight Hemophagocytic Lymphohistiocytosis (FIGHT HLH) program. Medical writing support was provided by Blair Hesp PhD CMPP of Kainic Medical Communications Ltd (Dunedin, New Zealand), which was funded by Sobi. Funding for this study was provided by Swedish Orphan Biovitrum AG, and by a grant from the European Commission through the First Targeted Therapy to Fight Hemophagocytic Lymphohistiocytosis (FIGHT HLH) programme.
Maria Ballabio and Cristina de Min are former employees of Swedish Orphan Biovitrum AB (publ).
Principal Investigator Statement: The authors confirm that the Principal Investigators for this paper are Prof. Michael B. Jordan and Prof. Franco Locatelli and that they had direct clinical responsibility for the patients.
Funding information European Commission Directorate‐General for Research & Innovation; Swedish Orphan Biovitrum AB (publ)
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to patient‐level data and related study documents including the study protocol and the statistical analysis plan. Patient‐level data will be de‐identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. To request access to clinical study data, please complete the data sharing request form and send together with any additional attachments to medical.info@sobi.com. Only duly completed data‐sharing request forms will be considered.
REFERENCES
- 1. Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041‐4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lehmberg K, Moshous D, Booth C. Haematopoietic stem cell transplantation for primary haemophagocytic lymphohistiocytosis. Front Pediatr. 2019;7:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henter JI, Samuelsson‐Horne A, Aricò M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH‐94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367‐2373. [DOI] [PubMed] [Google Scholar]
- 4. Trottestam H, Horne A, Aricò M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long‐term results of the HLH‐94 treatment protocol. Blood. 2011;118(17):4577‐4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Humblet‐Baron S, Franckaert D, Dooley J, et al. IFN‐gamma and CD25 drive distinct pathologic features during hemophagocytic lymphohistiocytosis. J Allergy Clin Immunol. 2019;143(6):2215‐2226.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergsten E, Horne A, Aricò M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long‐term results of the cooperative HLH‐2004 study. Blood. 2017;130(25):2728‐2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ehl S, Astigarraga I, von Bahr Greenwood T, et al. Recommendations for the use of etoposide‐based therapy and bone marrow transplantation for the treatment of HLH: Consensus statements by the HLH Steering Committee of the Histiocyte Society. J Allergy Clin Immunol Pract. 2018;6(5):1508‐1517. [DOI] [PubMed] [Google Scholar]
- 8. Buatois V, Chatel L, Cons L, et al. Use of a mouse model to identify a blood biomarker for IFNγ activity in pediatric secondary hemophagocytic lymphohistiocytosis. Transl Res. 2017;180:37‐52.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104(3):735‐743. [DOI] [PubMed] [Google Scholar]
- 10. Pachlopnik Schmid J, Ho CH, Chrétien F, et al. Neutralization of IFNgamma defeats haemophagocytosis in LCMV‐infected perforin‐ and Rab27a‐deficient mice. EMBO Mol Med. 2009;1(2):112‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henter JI, Elinder G, Söder O, Ost A. Incidence in Sweden and clinical features of familial hemophagocytic lymphohistiocytosis. Acta Paediatr Scand. 1991;80(4):428‐435. [DOI] [PubMed] [Google Scholar]
- 12. Xu XJ, Tang YM, Song H, et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr. 2012;160(6):984‐990.e1. [DOI] [PubMed] [Google Scholar]
- 13. Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89(2):207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bracaglia C, de Graaf K, Pires Marafon D, et al. Elevated circulating levels of interferon‐γ and interferon‐γ‐induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2017;76(1):166‐172. [DOI] [PubMed] [Google Scholar]
- 15. Prencipe G, Caiello I, Pascarella A, et al. Neutralization of IFN‐γ reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J Allergy Clin Immunol. 2018;141(4):1439‐1449. [DOI] [PubMed] [Google Scholar]
- 16. Hatterer E, Richard F, Malinge P, et al. Investigating the novel mechanism of action for NI‐0501, a human interferon gamma monoclonal antibody. Cytokine. 2012;59(3):570. [Google Scholar]
- 17. Locatelli F, Jordan MB, Allen C, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382(19):1811‐1822. [DOI] [PubMed] [Google Scholar]
- 18. US Food and Drug Administration. Denter for Drug Evaluation Research. BLA Multi‐Disciplinary Review and Evaluation. BLA 761107. GAMIFANT (emapalumab). 20 March 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761107Orig1s000MultidisciplineR.pdf. Accessed 10 November 2021.
- 19. Henter JI, Horne A, Aricó M, et al. HLH‐2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124‐131. [DOI] [PubMed] [Google Scholar]
- 20. Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ (Eds). NONMEM user‘s guides. Ellicott City, MD, USA: Icon Development Solutions; 1989. ‐2011. [Google Scholar]
- 21. Bergstrand M, Kalsson MO. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009;11(2):371‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alexander SPH, Christopoulos A, Davenport AP, et al. The Concise Guide to Pharmacology 2021/2022: G protein‐coupled receptors. Br J Pharmacol. 2021;178(S1):S264‐S312. [DOI] [PubMed] [Google Scholar]
- 23. Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to Pharmacology 2021/2022: Catalytic receptors. Br J Pharmacol. 2021;178(S1):S27‐S156. [DOI] [PubMed] [Google Scholar]
- 24. Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015;26(3):311‐327. [DOI] [PubMed] [Google Scholar]
- 25. Brice GT, Graber NL, Hoffman SL, Doolan DL. Expression of the chemokine MIG is a sensitive and predictive marker for antigen‐specific, genetically restricted IFN‐gamma production and IFN‐gamma‐secreting cells. J Immunol Methods. 2001;257(1‐2):55‐69. [DOI] [PubMed] [Google Scholar]
- 26. Chen P, Vu T, Narayanan A, et al. Pharmacokinetic and pharmacodynamic relationship of AMG 811, an anti‐IFN‐γ IgG1 monoclonal antibody, in patients with systemic lupus erythematosus. Pharm Res. 2015;32(2):640‐653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1Study designs
Supporting Information Table S2 Clinical and laboratory criteria to guide dose increases in the pivotal phase 2/3 study (Study NI‐0501‐04) [1]
Supporting Information Table S3 Covariate analysis and selection in PK models
Supporting Information Table S4 Baseline characteristics of healthy subjects (phase 1 NI‐0501‐03 study; A) and patients with HLH (phase 2/3 NI‐0501‐4/NI‐0501‐05 studies and patients with compassionate use experience; B)a
Supporting Information Table S5 Parameter estimates for the final popPK model in healthy subjects
Supporting Information Table S6 Parameters applied to data from healthy subjects administered emapalumab in a phase 1 study (Study NI‐0501‐03) when calculating the first dose of emapalumab in the pilot phase 2 study (Study NI‐0501‐04) in patients with primary HLH
Supporting Information Table S7 Comparison of model parameters of the base model (RUN506b) and the same model with covariate effect of corticosteroid doses (RUNDEXGAM) using data from patients with HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate use
Supporting Information Table S8 Summary statistics of the ROC analyses for the CXCL9 biomarkers resulting in a significant discrimination between patients with HLH with and without a clinical response using data from patients administered emapalumab in studies NI‐0501‐04/−05
Supporting Information Table S9 Summary statistics of the univariate logistic regression analysis of the clinical response at the end of treatment among patients with HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate use
Supporting Information Table S10 Summary statistics of the univariate logistic regression analysis of the AEs related to emapalumab exposure parameters among patients with HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate use
Supporting Information Figure S1 NI‐0501‐04 and NI‐0501‐05 original study schemas and subsequent amendments
Supporting Information Figure S2 Exploratory graphical analysis of plasma emapalumab concentrations on a semi‐log scale for individual healthy subjects over time (Study NI‐0501‐03)
Supporting Information Figure S3 Predictions of emapalumab concentration‐time profiles after the first administration of 1 mg/kg in patients with HLH of 23 kg with various levels of Vmax a
Supporting Information Figure S4 Goodness‐of‐fit plots presenting (A) observations versus population predictions and (B) observations versus individual predictions using the final popPK model for patients with primary HLHa
Supporting Information Figure S5 Visual predictive check of simulated versus observed plasma emapalumab concentrations over time (A) and by total IFNγ concentration (B)a
Supporting Information Figure S6 Inhibition by emapalumab of the IFNγ‐induced CXCL9 productiona
Supporting Information Figure S7 CXCL9 concentrations as a function of time after the first emapalumab administration in patients with primary HLH participating in the pilot phase 2 study (Study NI‐0501‐04; N = 50)
Supporting Information Figure S8 Effect of glucocorticoid doses on CXCL9 steady‐state concentrations at increasing emapalumab concentrations based on data derived from patients with primary HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate use
Supporting Information Figure S9 Goodness‐of‐fit plots of CXCL9 derived from popPK‐PD analyses from patients with HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate usea
Supporting Information Figure S10 Visual predictive checks of RUN506b. Simulated CXCL9 concentrations as a function of emapalumab concentrations (left graph)a and total IFNγ concentration (right graph) versus observed values from patients with HLH administered emapalumab in studies NI‐0501‐04/−05 or for compassionate use
Supporting Information Figure S11 Examples of concentration‐time profiles of free emapalumab (blue, left log axis), total IFNγ (red, left log axis), model derived free IFNγ (black, left log axis) and CXCL9 (green, right log axis) for individual patientsa
Supporting Information Figure S12 Simulated CXCL9 concentration‐time profiles for individual patients with HLH and differing bodyweight or total IFNγ levels administered emapalumab 1‐10 mg/kg
Data Availability Statement
Qualified researchers may request access to patient‐level data and related study documents including the study protocol and the statistical analysis plan. Patient‐level data will be de‐identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. To request access to clinical study data, please complete the data sharing request form and send together with any additional attachments to medical.info@sobi.com. Only duly completed data‐sharing request forms will be considered.


