FIGURE 1.
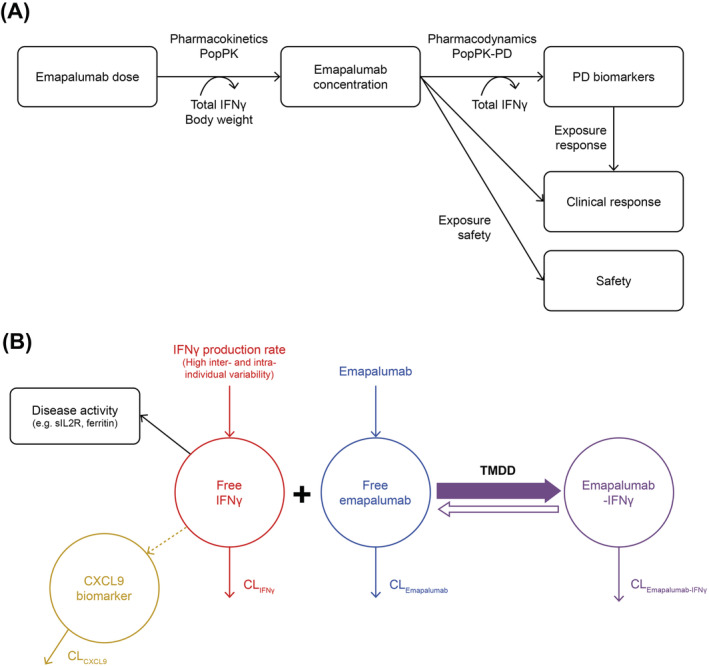
Schematic representation of (A) the overall PK‐PD‐efficacy/safety analysis strategy for emapalumab in patients with HLH and (B) PK‐PD‐disease interactions between IFNγ, emapalumab, CXCL9 and HLH disease activity. Note: In patients with HLH, the endogenous production of IFNγ (red) is exacerbated and leads to high circulating concentrations of free IFNγ. After administration, emapalumab (blue) binds reversibly to IFNγ, reducing its free concentration, and leads to the formation of an IFNγ‐emapalumab complex (violet), which is in equilibrium with the free moieties. To confirm the neutralisation of IFNγ by emapalumab, the decrease in serum concentration of CXCL9 (orange), a chemokine almost exclusively produced through the activation of the IFNγ receptor, is monitored. As such, CXCL9 can be considered as a biomarker of free IFNγ activity, hence the pharmacological activity of emapalumab in neutralising IFNγ. The decrease in free IFNγ is expected to improve disease status, which can be monitored by parameters such as sIL2R and ferritin (black). These parameters are included in the panel of HLH diagnostic criteria and considered as components of response to HLH treatments. All components described above (ie, free IFNγ, free emapalumab, IFNγ‐emapalumab complex, CXCL9, sIL2R and ferritin) have their own intrinsic clearance and their concentrations correspond to an equilibrium between production and elimination. Furthermore, for emapalumab, the formation of the complex with IFNγ corresponds to an additional clearance proportional to the IFNγ production. This phenomenon is known as TMDD. In patients with HLH receiving emapalumab, serum concentrations of free emapalumab (before drug administration), total IFNγ (ie, free IFNγ + IFNγ‐emapalumab complex) and CXCL9 have been measured. When free emapalumab is in excess, total IFNγ concentration at equilibrium can be considered as proportional to the IFNγ production and changes in total IFNγ concentration indicate proportional changes in IFNγ production. CL, clearance; CXCL9, chemokine (C‐X‐C motif) ligand 9; IFNγ, interferon γ; HLH, haemophagocytic lymphohistiocytosis; PD, pharmacodynamic; PK, pharmacokinetic; Pop, population; sIL2R, soluble interleukin‐2 receptor; TMDD, target mediated drug disposition
