FIGURE 4.
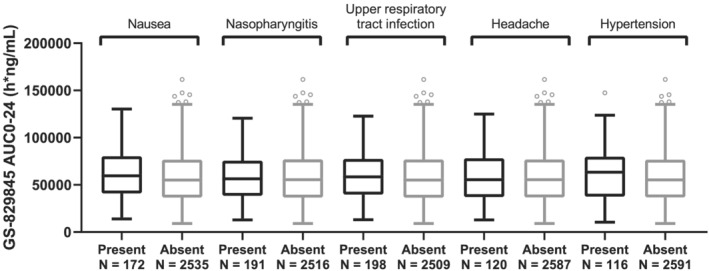
GS‐829845 AUC0‐24 by the five most frequent treatment‐emergent adverse events in subjects with rheumatoid arthritis (RA) up to week 52 data. The analysis set includes subjects with RA who were enrolled/randomized, received at least one dose of filgotinib in studies FINCH 1, FINCH 2, FINCH 3, DARWIN 1, DARWIN 2 and DARWIN 3, and had at least one nonmissing pharmacokinetic (PK) parameter of interest. For each box, the bottom and top edges are located at the sample 25th (Q1) and 75th (Q3) percentiles, respectively, the centre horizontal line is drawn at the 50th percentile (median) and outliners (beyond 1.5 × the interquartile range) are displayed as small squares. AUC0‐24 is the population PK‐predicted exposure in phase 2 and phase 3 subjects with RA receiving filgotinib
