Figure 2.
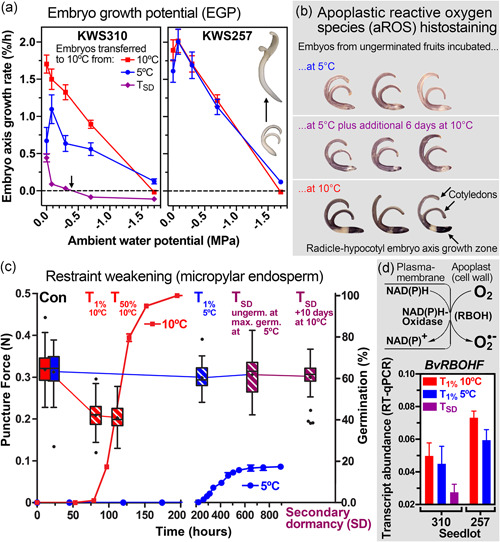
Inhibition of embryo growth and endosperm weakening associated with the cold‐induced secondary dormancy of sugar beet. (a) Embryo growth potential (EGP) analysed as embryo axis elongation growth of isolated embryos under different conditions of temperature and water potential (n ≥ 13). Embryos were extracted from KWS310 fruits either at 5°C or 10°C at the respective T 1% or from secondary dormant fruits (T SD) and incubated at the indicated water potential (see Figure S5 for details). Note that the growth potential of embryos from secondary dormant fruits was severely reduced (arrow marks threshold water potential where growth ceases). The contrasting lot KWS257 (Figure S2) served as comparison. (b) Histochemical analysis of apoplastic reactive oxygen (aROS) using the NBT histostain for superoxide with embryos extracted from KWS310 fruits imbibed in different temperature regimes. Compare the intense aROS histostain in the growing zone (hypocotyl‐radical axis) of 10°C embryos with 5°C and upon secondary dormancy. (c) Biomechanical analysis of KWS310 micropylar endosperm weakening. Puncture force (tissue resistance) of micropylar endosperm tissue of sugar beet fruits imbibed at the two temperature regimes (5 vs. 10°C) and during secondary dormancy (T SD); the overlay shows the germination kinetics. Con, control early timepoint; T 1%, physiological timepoints at which 1% of the population has germinated; T 50%, the timepoint at which half the population has germinated; T SD (ungerminated fruits analysed at maximum germination at 5°C), timepoint at which cold‐induced secondary dormancy has been induced during the imbibition at 5°C; T SD (+10 days at 10°C), timepoint during secondary dormancy maintenance for the subpopulation of ungerminated fruits, which has been transferred from 5°C for 10 days incubation at 10°C. Note that a rapid decrease in endosperm tissue resistance (puncture force) occurred before the completion of germination at 10°C, whereas this endosperm weakening was blocked at 5°C and during secondary dormancy fruits. Mean values, medians and whisker box plots are presented, n ≥ 29. (d) Transcript abundances (RT‐qPCR) of the aROS producing NAD(P)H oxidase BvRBOHF in KWS310 and KWS257 sugar beet seeds at their respective T 1% timepoints at 5°C (blue), 10°C (red) and T SD (purple); for a detailed BvRBOHF analysis, see Figure S8. Mean values ± SEM are presented [Color figure can be viewed at wileyonlinelibrary.com]
