Abstract
Immunotherapy has revolutionized treatment for many hard-to-treat cancers but has yet to produce significant improvement in outcomes for patients with glioblastoma. This reflects the multiple and unique mechanisms of immune evasion and escape in this highly heterogeneous tumor. Glioblastoma engenders profound local and systemic immunosuppression and is remarkably effective at inducing T-cell dysfunction, posing a challenge to any immunotherapy-based approach. To overcome these mechanisms, multiple disparate modes of immune-oriented therapy will be required. However, designing trials that can evaluate these combinatorial approaches requires careful consideration. In this review, we explore the immunotherapy resistance mechanisms that have been encountered to date and how combinatorial approaches may address these. We also describe the unique aspects of trial design in both preclinical and clinical settings and consider endpoints and markers of response best suited for an intervention involving multiple agents.
Introduction
Patients with glioblastoma survive for 12 to 15 months on average despite treatment with surgery, focal irradiation, alkylating chemotherapy, and tumor treating fields (1–4). Although several immunotherapies are currently under investigation for glioblastoma, none have yet demonstrated a significant survival benefit (5–7). Glioblastoma originates in an immune privileged compartment and is adept at escaping immune surveillance (8). Precision immunotherapy also requires a uniformly expressed tumor-specific antigen (TSA), which remains elusive in highly heterogeneous isocitrate dehydrogenase (IDH) wild-type gliomas or glioblastoma (9). Further, glioblastoma disrupts immune function both locally and systemically, degrading the ability of immunotherapy to act (10).
Given these significant obstacles, it is clear that a single agent will be insufficient. To unlock the true potential of immunotherapy, combinations with additive and/or synergistic mechanisms of action are required. However, testing these combinations poses unique technical, logistical, and regulatory challenges. In this review, we will explore current opportunities and describe strategies for conducting trials of combination immunotherapy.
Current and Future Combinatorial Strategies
Glioblastoma induces immune dysfunction through multiple mechanisms (10–15). To overcome these, several immunotherapy classes are under investigation, including immune checkpoint blockade (ICB), chimeric antigen receptor T cells (CAR-T), bi-specific T-cell engagers (TCE), tumor antigen vaccination, oncolytic viruses, and immunomodulatory cytokines (16–21). Given the large number of permutations for combination therapy, we must rationalize the available options.
Combinations of multiple immune checkpoint blockade
Single-agent ICB has failed to yield benefit in patients with glioblastoma (22). Given this, studies have been performed using multiple ICB agents, based on effective strategies in other difficult malignancies such as melanoma or advanced renal cell carcinoma (23). A well-described combination in oncology is that of nivolumab (anti–PD-1) and ipilimumab (anti–CTLA-4), which has been explored in multiple trials (NCT02017717 – Checkmate 143, NCT04145115, NCT03233152, NCT04003649, NCT03422094, NCT02311920, and NCT03707457). However, subsequent work has demonstrated that glioblastoma exhibits cancer lineage–specific resistance to the reversal of T-cell exhaustion, which may reduce the impact of this particular combination (24). Retrospective genomic and transcriptomic analysis of patients who received PD-1 inhibitors found that the degree of response to treatment was associated with specific evolutionary pathways resulting in certain molecular and immune expression profiles. This would indicate that only certain subsets of patient may benefit from this form of checkpoint blockade (25). The timing of checkpoint blockade therapies relative to standard-of-care treatment also may play a key role in efficacy. Cloughesy and colleagues report upregulated T-cell and IFNγ gene expression and downregulated cell-cycle gene expression within the tumor when anti–PD-1 therapy was used in the neoadjuvant setting. This effect was however not observed for those patients that received adjuvant therapy alone, suggesting a transient window of opportunity for checkpoint blockade (26). Schalper and colleagues also found positive immune effects associated with neoadjuvant PD-1 blockade, reporting enhanced levels of immune-cell infiltration and greater TCR diversity among tumor-infiltrating lymphocytes (TIL), suggesting that this may be a useful partner with other immunotherapies (27).
Other checkpoint inhibitory molecules highly expressed by TILs in glioblastoma include indoleamine 2,3-doxygenase (IDO1), T-cell immunoglobulin-mucin-domain containing-3 (TIM-3), and lymphocyte activation gene 3 (LAG3; ref. 28). Phase I clinical trials exploring combination approaches against both PD-1, LAG-3 (NCT02658981), and IDO1 (NCT03707457) are now underway. Despite this, experience of combinatorial PD-1 and IDO1 blockade in the central nervous system (CNS; albeit in metastatic melanoma) have failed to improve outcomes in phase III studies (29). Other combination approaches using IDO1 inhibitors with temozolomide (TMZ; NCT02052648) remain under evaluation in glioblastoma. Although these findings may suggest that combinatorial ICB may still struggle in the CNS, lack of success may actually reflect incomplete checkpoint blockade. Opitz and colleagues have described metabolic pathways in glioma such as activation of the aryl hydrocarbon receptor (AHR) by tryptophan catabolites, which results in enhanced malignancy and immunosuppression (30). Although the AHR pathway was initially associated with IDO1 or the tryptophan-2,3-dixoygenase 2 (TDO2) enzyme, recent work has demonstrated that IL4-induced-1 (IL4I1) is more significantly associated with AHR activity. ICB can induce IDO1 and IL4I1, whereas IDO1 inhibitors previously trialed in combination with ICB do not result in IL4I1 blockade (31). Future combinatorial pairings of ICBs should consider the potential for anti–PD-1 agents to induce metabolic agents which upregulate metabolic pathways of immunosuppression.
Immune checkpoint blockade and T-cell–directed immunotherapy
Intratumoral heterogeneity in glioblastoma poses a significant barrier to antigen-specific immunotherapies such as CAR-T cells or bispecific T-cell engagers. CAR-T cells specific for EGFR variant III (EGFRvIII) have proven ineffective when treating recurrent tumors due to antigen escape (9). The intended selective targeting of cells or spontaneous elimination of target cells at recurrence produces an outgrowth of antigen-negative cells resulting in recurrence (9, 32, 33). A high degree of clonality and, contrary to other cancers, a high mutational burden in glioblastoma has also been associated with resistance to ICB (34, 35). Of note for clinical trials, high tumor mutational burden can be induced by TMZ, which causes defects in DNA mismatch repair genes. One potential approach to overcoming this is by targeting IDH1-R132H, a shared clonal neo-epitope in IDH-mutated gliomas. This uniformly expressed TSA in a subset of patients with glioma has been successfully targeted in recent phase I trials (NCT02454634), and was found to be both safe and immunogenic (36).
Although prior phase Ib trials (NCT02287428) of neoantigen vaccination in glioblastoma have reported neoepitope-specific systemic immune responses with increased numbers of TILs, these have also been shown to express a profoundly exhausted phenotype (37). Combining ICB with a vaccine strategy targeting a shared clonal neoepitope may therefore work synergistically to overcome ICB resistance while enhancing the neo-epitope immune response. This is supported by preclinical evaluation of multivalent neoantigen vaccines with ICB, which generated greater antitumor responses than monotherapy, even in models with reduced anti–PD-L1 sensitivity (38, 39). Such an approach would therefore be logical to evaluate for other multi-epitope vaccine-based approaches such as that used in the GAPVAC trial (NCT02149225; ref. 40). As mentioned previously, neoadjuvant anti–PD-1 blockade has been associated with enhanced clonal expansion of T cells and greater immune infiltration/TCR diversity (26, 27). This would also likely benefit immunotherapy approaches that rely heavily on T-cell expansion such as vaccination or CAR-T cell therapy. Further, CAR-Ts targeting EGFRvIII have been shown to upregulate expression of PD-L1 within gliomas, contributing to CAR-T cell dysfunction and treatment failure (9). The addition of anti–PD-1 blockade to such approaches may therefore increase both the diversity and potency of the immune response to CAR-T therapy while reducing T-cell exhaustion. This is supported by work by Choi and colleagues who designed a CRISPR-Cas9 modified EGFRvIII CAR-T cell with the endogenous PD-1 receptor knocked out, thereby preventing PD-L1 binding. This CAR-T–EGFRvIII PD-1 construct resulted in prolonged survival in mice bearing EGFRvIII+ glioma compared with CAR-T–EGFRvIII cells with an intact PD-1 receptor (41). In this vein, trials are underway evaluating CAR-T cell therapy (NCT04003649) and vaccination (NCT04201873, NCT02529072, NCT02287428) alongside ICB. Newer trial designs are also being deployed such as the AMPLIFY-NEOVAC surgical window-of-opportunity trial (NCT03893903). This will evaluate IDH1R132H vaccination with avelumab (anti–PD-L1) to explore predictive biomarkers for response to ICB in patients with IDH-mutated gliomas.
It is notable that studies such as that performed by Choi and colleagues report prolonged survival with direct intracerebral or intraventricular delivery of CAR-T therapy but that this therapeutic effect is lost with peripheral administration. This finding serves to demonstrate that transiting the blood–brain barrier (BBB) remains a formidable obstacle for many systemically delivered immunotherapies (42). Even in the pathologic glioma state, regions of the BBB likely remain intact, shielding sections of tumor from immunotherapy, which may then act as the focal point for recurrence (43). Although systemic anti–PD-1 therapy has been noted to induce changes in the CNS, it is unclear where this interaction with the immune system occurs and indeed what concentration is necessary to induce an effect at the intracranial tumor site (44). Although one solution may be direct intracranial delivery of agents, this highly invasive approach will not be suitable for all patients and faces significant challenges in achieving equal and persistent drug distribution throughout the tumor (33, 45, 46). Another potential approach may be the use of ex vivo activated autologous T cells combined with T-cell engaging therapies. These activated T cells would theoretically adhere to the brain microvascular endothelium and traffic into the brain, carrying their immunotherapy payload on their surface (18, 47). However, this effect has also been associated with neurotoxicity and must therefore be investigated with caution (48). Accordingly, such an approach is entering phase I safety trials where a hEGFRvIII-CD3 Brain Bi-Specific T Cell Engager (BRiTE) will be evaluated alongside peripheral autologous T-cell infusion (NCT04903795).
Other strategies to enhance the T-cell repertoire and overcome immunosuppression
Combination of immunotherapy with radiotherapy has been demonstrated in melanoma to expand the compartment of effector memory T cells and TILs, while also inducing a more diverse T-cell receptor population when combined with ICB (49). Similar promise has been demonstrated preclinically in glioblastoma, where TIM-3 and PD-1 antibodies combined with radiotherapy achieved long-term survival (50). Other strategies to expand TCR diversity may involve the use of dendritic-cell vaccines, which carry antigen from the tumor to draining lymph nodes, presenting them to effector T cells. Chemokines such as the macrophage inflammatory protein-1 alpha (MIP-1α, CCL3) may aid in enhancing lymph node chemotaxis of dendritic-cell subsets both to tumor and from tumor to lymph node, resulting in greater diversity of antigen presentation and more potent antigen-specific T-cell responses (51). Dendritic cells may also enhance the polyfunctionality of adoptively transferred T cells targeting tumor-specific antigens in glioblastoma (52).
Enhancing T-cell functionality may be supported by the use of costimulatory agonists such as CD27, 4-1BB, OX40, or CD40, which are now entering early clinical trials (e.g., NCT04547777, NCT02658981, NCT03688178; refs. 53–56). Newer constructs that combine both anti-inhibitory and prostimulatory strategies are under development such as bispecific antibodies targeting both CD27 and PD-L1 (NCT04440943) or TGFβ and PD-L1 (57). Novel CAR-T constructs including synNotch and armored CARs with expression of cytokines such as IL12 have also been demonstrated to enhance antitumor efficacy in the context of oncogenic immunosuppression (58, 59). When considering immunosuppression, thought should also be given to the role of dexamethasone, which may induce systemic depletion of memory and naïve CD4/CD8 T cells, reducing the efficacy of immunotherapy (60). In this context, agents that have failed to show efficacy when combined with checkpoint blockade such as bevacizumab (anti-VEGF) may also be worth re-evaluating as an adjunct, specifically for its ability to reduce the need for immunosuppressive corticosteroids (61, 62).
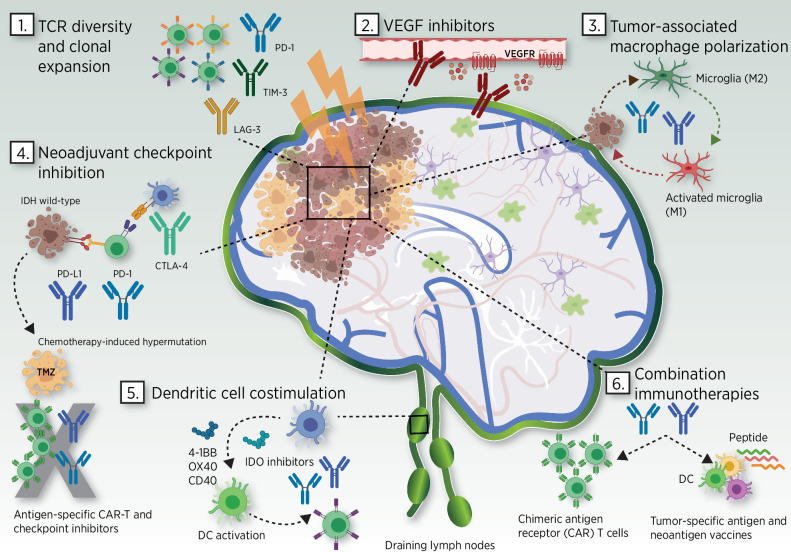
A summary of potential combinatorial approaches is depicted in Fig. 1. However, as described above, enhanced additive synergism between immunotherapies may well extend beyond a bimodal approach. It is reasonable to consider trial designs, which involve three or more elements. This will require flexible trial designs to swiftly identify the optimal combinatorial schedule which are discussed in the following section.
Figure 1.
Combination approaches using checkpoint inhibition and other therapies for glioblastoma. 1. TCR diversity and clonal expansion. Combination radiation therapy and inhibitors of TIL exhaustion and drivers of apoptosis (PD-1, LAG-3, TIM-3) are being studied for synergistic effects on TIL expansion and clonal diversity. 2. VEGF inhibitors. Anti-VEGF therapies such as bevacizumab are being utilized as steroid-sparing agents to harness immunotherapy-related toxicity in the CNS. 3. Tumor-associated macrophage polarization. Glioma cells interact with and maintain a robust population of PD-1–expressing microglia with an anti-inflammatory phenotype (M2). Selective anti–PD-1 blockade on microglia populations is capable of inducing a tumoricidal M1 phenotype (87–89). 4. Neoadjuvant checkpoint inhibition. Treatment-naïve, IDH wild-type glioblastoma upregulates PD-L1 and CTLA-4 offering enhanced sensitivity to immune checkpoint combination approaches. Neoadjuvant checkpoint blockade increases clonal expansion of T cells. Chemotherapy with temozolomide can alter tumor mutational burden resulting in both increased resistance to checkpoint blockade and increased subclone heterogeneity thus limiting the potency of antigen-specific immunotherapies such as CAR-T cells. 5. Dendritic cell costimulation. Costimulatory agonists for 4–1BB, OX40, and CD40 and IDO inhibitors are being evaluated with checkpoint inhibitors to polarize cytotoxic T-cell responses in the tumor microenvironment and within immunosuppressed tumor-draining lymph nodes. 6. Combination immunotherapies. Multivalent neoantigen vaccines and CAR-T cell therapies in combination with checkpoint inhibitors are being evaluated for superior efficacy compared with single modalities even with reduced PD-L1 sensitivity. Abbreviations: CAR, chimeric antigen receptor; CTLA-4, cytotoxic T-lymphocyte–associated protein 4; DC, dendritic cell; IDO, indoleamine 2, 3-dioxygenase; LAG-3, lymphocyte activation gene 3 (LAG3); M1 and M2, macrophage pro-inflammatory and anti-inflammatory phenotype; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TCR, T-cell receptor; TIM-3, T-cell immunoglobulin-mucin-domain containing-3; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor. Adapted from an image created with BioRender.com.
Recommendations for Combinatorial Clinical Trial Design
Population selection
When considering any new combinatorial therapy, a proof-of-principle study is necessary to determine efficacy. Outcome measures in this context usually consist of specific biologic endpoints. However, these studies are typically performed on small numbers of patients with late-stage disease who have received highly variable treatment courses. This can make interpretation of said biologic endpoints difficult. Selecting patients earlier in the disease course with a less heterogeneous and slower growing pathology may make interpretation of biological markers easier, while also allowing combination immunotherapies sufficient time to synergize and induce maximal biological effect. Particular consideration should also be given to patients with an unmethylated O6-methylguanine-DNA methyltransferase (MGMT) promoter gene, who are less likely to respond to TMZ. In such cases, omission of TMZ entirely would be clinically justifiable and would allow evaluation of new therapies without additional toxicities from TMZ or inducing hypermutation as described previously (63, 64).
Regimen selection
The optimal dosing schedule may be extremely broad when designing trials for two or more agents and is further complicated by the fact that true synergy may exist at nonmaximal doses. Although it is generally not acceptable to reduce doses of standard-of-care agents in combination, an appreciation for the unique pharmacodynamic interplay between combined immunotherapy agents is required. Although this might suggest a need for extensive preclinical testing, newer designs such as phase 0, translational, surgical “window-of-opportunity”, or neoadjuvant trials may offer a route to bypass potentially laborious steps (65, 66).
Phase 0 trials use a microdosing strategy to allow for assessment of potential pharmacodynamic (PD) and pharmacokinetic (PK) properties while minimizing risk. This allows for rapid determination of the biological activity of a potential combination and allows for early termination if said combination does not meet its predefined PD/PK endpoints. Window-of-opportunity studies take an alternative route, using a predefined therapeutic dose and typically aim to define target engagement and/or immune modulatory endpoints. Both trial approaches aim to determine biological and immunomodulatory impact, rather than clinical effect, although window-of-opportunity trials also allow for correlation with eventual patient outcomes. Although these are both useful approaches for evaluating new combinations, perhaps the most sensitive way to determine synergism between two agents is by using neoadjuvant studies in which treatment is administered preoperatively and tumor samples are taken for analysis at time of resection.
Neoadjuvant studies have already been used successfully to determine the effect of immune checkpoint inhibition on the intratumoral T-cell compartment (26). The neoadjuvant approach may also be superior to postsurgical biopsies of tissue, which are prone to sampling error and often have limited tissue availability, resulting in a nonrepresentative immune analysis. A large volume of tissue will allow for not just a determination of raw numbers of TILs but functional activity. Such analyses would be superior to peripheral immune interrogation, which may not be equivalent to events occurring at the tumor site.
Moving beyond phase I
If a combination proves safe and tolerable while also demonstrating evidence of immune response, trials should proceed to phase II trials to assess clinical benefit. However, traditional single-arm phase II trials often use response rates (RR) based on historical controls as the main endpoint, which may be inappropriate for combined immunotherapy. An efficient approach would be to use seamless phase I/II, II/III clinical trials, whereby the protocol specifies when to transition the study from a certain phase (e.g., phase II to III) without the need for a new protocol or regulatory process (67, 68). Similarly, large phase Ib trials and “expansion baskets” of the combination in phase I trials allow for increasing the number of patients enrolled once the recommended phase II dose has been determined. This allows for the phase I study to aid establishing preliminary efficacy in addition to determining the safety of the combination. Basket and platform trial designs using master protocols allow for within-basket immune monitoring depending on the approaches being evaluated (e.g., markers of T-cell activation if blocking regulatory T-cell receptors; ref. 69). The use of adaptive designs in this setting allows for adjustment according to evolving data so that poorly performing combinations can be abandoned early, while additional treatment cohorts which test other combinations can be added.
Many basket trials to date (Lung-MAP, NCT02154490; NCI-MATCH, NCT02465060; and My Pathway, NCT02091141) use RR to evaluate targeted therapies, as is typical for traditional single-arm early-phase trials. However, this may not be an appropriate endpoint for the evaluation of immunotherapies, which can yield clinical benefit without a high RR. Indeed, immunotherapies have been noted to induce imaging changes interpreted as indicative of progression (70). To address this, adaptive designs have been initiated including the INSIGhT adaptive platform trial (NCT02977780), the glioblastoma AGILE phase II/III adaptive platform trial (NCT03970447; refs. 64, 71) in the United States, and the NCT neuro master match umbrella phase I/IIa trial (NCT03158389) in Germany. Overall survival (OS) can be used in these trials as the primary endpoint rather than RR (72, 73). These designs are mostly used to evaluate targeted therapies to circumvent lengthy pauses between trial phases, but their usage still lags behind for immunotherapy-based approaches (74).
Recommendations for Ensuring Safety and Determining Outcomes
In the context of combinatorial immunotherapy, toxicity considerations are complicated by the need to determine the appropriate regimen for commencing two or more agents at once. Multiple agents may act synergistically in both efficacy and toxicity, which could result in potentially fatal complications such as cytokine release syndrome (CRS; ref. 75). Model-assisted designs are a useful tool to assess the prestudy probability of toxicity and can inform dose-escalation decisions using real-time adverse event data (76, 77). These can maximize the number of patients treated at or near the MTD and outperform traditional designs such as 3 + 3 dose escalation, which have yielded inconsistent dose-toxicity or dose-escalation correlations (78, 79). However, although the flexibility and accuracy of model-assisted designs may be of particular use when evaluating immunotherapy combinations, these require sustained biostatistical collaboration, which can be time and resource intensive. A detailed evaluation of the dose-escalation strategy and how best to determine response is therefore vital before commencing clinical trials.
Determining the starting dose
Many combinatorial trials use agents with a known safety profile and where the biologically active dose is known. For that reason, such trials could begin at the optimal dose rather than using conservative escalations. When neither component is known to be effective or approved for the indicated use, and when neither will be effective alone, a two-arm design comparing combination to a control agent (or placebo) can be considered (shown in Fig. 2). However, although this minimizes exposure of patients to inactive therapies, this design may not demonstrate the inactivity of specific components. When the safety profile or optimal dose is unknown for either component in a combination, the optimal immune response may be an appropriate endpoint for early-phase trials, providing there are no significant toxicities. However, establishing response with multiple agents and titrating each element to maximize efficacy may not be practical.
Figure 2.
Rationalizing trial designs for combinatorial immunotherapy. Regulatory approval of combinations of therapeutic agents in medicine usually requires a demonstration of each component's independent contribution. The ability to evaluate pharmacodynamic effects of single agents and/or combinations may help determine whether randomized studies require arms including both single agents and combinations. When neither component is known to be effective or approved for its indicated use, or when neither have efficacy as a single agent by itself, a two-arm design comparing the combination to a control agent (placebo) can be considered. Assessment of immune response must not only quantify the degree of immune activation but also the functional status of the response generated. Adapted from an image created with BioRender.com.
Measuring effect
Patients in phase I trials are likely to have relapsed or progressed on previous therapies but preliminary signals of activity can still be noted using RR, progression-free survival (PFS), recurrence-free survival, or OS. However, in an advanced-disease population, immunotherapies may not induce a strong immune effect that manifests as a reduction in disease burden. Patients may also not survive long enough to have time to generate the immune response that would provide a clinical benefit. A further complication is that OS can be extended by immunotherapy without radiologic response or pseudo-progression (80). The modified response assessment in neuro-oncology (mRANO) and immune-specific RANO (iRANO) aim to standardize determination of response, but their utility in trials using experimental combinations is yet to be validated (70). To determine response more accurately and avoid premature treatment discontinuation, the definition of progression will need to require confirmation on two separate observations or to not consider early progression within a prospectively defined time-interval as per modified RANO (81). In patients who do respond after early progression, PFS should be based on the start of therapy.
Determining potency, predicting clinical effects, and understanding the impact of the manufacturing process on the final drug product and stability are all required for regulatory approval. However, demonstrating these effects in combination therapies poses practical and ethical problems. Evaluation of purity and potency can be difficult if a treatment is composed of a combination of heterogeneous components (e.g., autologous blood or tumor-derived cellular therapies; ref. 82). One approach to overcome this is to use quantitative assessments such as the time to kill 50% of target tumor cells (KT50; ref. 83). Other approaches may involve correlating outcomes with serial immune assays to quantitatively measure the relative immunogenicity of a combination. Statistical modeling using toxicity and antitumor toxicity have also been considered (84, 85). However, many of the techniques used including cytokine release, tetramer, cytotoxicity, and the enzyme-linked immune absorbent spot (ELISpot) assays are often only technically validated in the research laboratory, and the frequency with which they are performed vary widely, leading to variable results (86).
To generate valid and transferable data based on immune assays, harmonization and standardization of techniques is required to establish the expected immune response from known effective immunotherapies, against which new combinations can be evaluated. Current FDA-approved biomarkers of tumor mutational burden (TMB) that are used to predict response to the checkpoint inhibition pembrolizumab in solid tumors, do not have the same predictive value in gliomas (35) and therefore a pathology-specific assay is required.
Conclusion
Although immunotherapy holds significant promise for overcoming the challenges of immune dysfunction and tumoral heterogeneity, it is increasingly apparent that a single agent alone will not suffice. Exploration of combinations of ICB with neo-epitope vaccination strategies in IDH mutant gliomas is one promising approach, but IDH is expressed in a minority of glioblastomas. Further, the cancer lineage–specific ability of glioblastoma to drive T-cell anergy and apoptosis poses a significant obstacle for ICB therapies. Given this, more work is required on alternative strategies such as combinatorial T-cell costimulation or blockade of tumoral metabolic pathways. Evaluating potential combinations in patients who may not benefit from TMZ and are therefore less prone to hypermutation will be helpful to accurately determine biological activity. The timing of administration relative to routine clinical interventions such as steroid administration, radiation therapy, and the aforementioned alkylating chemotherapy, all of which possess varying immuno-modulatory effects, must also be weighed. Neoadjuvant and surgical window of opportunity studies (where tumor tissue can be collected after combinatorial immunotherapy) may offer the most sensitive PD and PK analysis, but other surrogate markers of effect such as KT50 are also useful. Model-assisted trial designs may help assess dosage and schedules for different combinations, but it is important to consider that maximal synergistic effect may not occur at the maximal therapeutic dose. Although combinatorial approaches may unlock the true potential for immunotherapy in glioblastoma, the lack of success in glioblastoma immunotherapy trials demands a tailor-made combinatorial approach. International collaboration will be necessary to develop trials, which have the scope and recruitment necessary to integrate such biologic complexities into their design (see summary box).
Summary Box and Key Messages
| To date, immune-based monotherapies have failed to improve survival of patients with glioblastoma. |
| Glioblastoma exerts cancer lineage–specific mechanisms of immune escape and can induce profound local and systemic immunosuppression. |
| Given the lack of efficacy seen when using combinations with anti–PD-1/anti–CTLA-4 to date, cancer lineage–specific checkpoint inhibition (e.g., IDO1, LAG3, TIM3) and costimulatory agonistic targets (e.g., CD40, CD27) are worth exploring. |
| Timing of combinatorial immunotherapy relative to standard-of-care treatment must be carefully weighed. |
| TMZ may induce hypermutation and drive heterogeneity, and drive resistance to immunotherapies and their combinations. |
| Bevacizumab may help to reduce edema and therefore reduce the need for immunosuppressive corticosteroids, and can be used as an adjunct to combination immunotherapy. |
| Harmonization, standardization of immune technologies, and generation of reference values will help accelerate preclinical and early clinical development in glioblastoma. |
| Flexible trials such as model-assisted and adaptive designs are required to rapidly assess potential novel combinations. |
| Surgical window-of-opportunity trials, neoadjuvant trials, and trials with primary biologic (PD, PK) endpoints are recommended as they may help shorten lengthy pre-clinical and often futile clinical investigation. |
Authors' Disclosures
P.Y. Wen reports personal fees from Agios, AstraZeneca/Medimmune, BeiGene, Bayer, Boston Pharmaceuticals, Celgene, Eli Lilly, CNS Pharmaceuticals, Genentech/Roche, Kazia, Medicinova, Elevate Bio Immunomic Therapeutics, Imvax, Karyopharm, Merck, Novartis, Nuvation Bio, Oncoceutics, Vascular Biogenics, VBI Vaccines, Voyager, Celularity, and Sapience outside the submitted work. A.C. Tan reports personal fees from Amgen outside the submitted work. S.J. Bagley reports grants from Incyte, Eli Lilly, and GSK; grants and personal fees from Novocure; and personal fees from Bayer and Sumitomo Dainippon outside the submitted work. S.J. Bagley also has a patent for Combination of EGFRvIII Chimeric Antigen Receptor T cells and PD-1 Inhibitor issued to University of Pennsylvania. M. Lim reports grants and other support from Arbor; grants from BMS, Merck, Accuray, Kyowa-Kyrin, and Incephalo; personal fees from Tocagen, VBI, Pyramid Bio, Insightec, Novocure, Sanianoia, Black Diamond, and Egret Therapeutics; and grants and personal fees from Biohaven and Hemispherian during the conduct of the study. In addition, M. Lin has a patent for Focused Radiation + Checkpoint Inhibitors issued and a patent for Local Chemotherapy + Checkpoint Inhibitor issued and licensed to Arbor. M. Platten reports personal fees from Bayer, Affiris, and Vaximm outside the submitted work; in addition, M. Platten has a patent for EP2753315B1 issued and with royalties paid from Bayer and a patent for EP2800580B1 issued. H. Colman reports personal fees from Best Doctors/Teladoc, Orbus Therapeutics, Adastra Pharmaceuticals, Bristol Meyers Squibb, Karyopharm Therapeutics, Newlink Genetics, AbbVie, Forma Therapeutics, Bayer, and Private Health outside the submitted work. D.M. Ashley reports personal fees from Diverse Biopharma, Immunogenesis, Medtronic, and Maia, Inc. during the conduct of the study, as well as personal fees from Diverse BioPharma, Immunogenesis, Medronic, and MAIA, Inc. outside the submitted work. S.M. Chang reports other support from Agios outside the submitted work. E. Galanis reports personal fees from Gradalis, Inc. and Kiyatec, Inc., as well as grants from Servier Phramaceuticals LLC (formerly Agios Pharmaceuticals, Inc.), Celgene, MedImmune, Inc., and Tracon Pharmaceuticals outside the submitted work. V.K. Puduvalli reports personal fees from Orbus Therapeutics, Ziopharm, and Novocure, as well as grants from Bexion, Prelude, and Karyopharm outside the submitted work; in addition, V.K. Puduvalli reports equity in Gilead Pharma and Amarin. D.A. Reardon reports personal fees from AbbVie, Advantagene, Agios, Amgen, Heart Therapeutics, Bayer, Boston Biomedical, Boehringer Ingelheim, Deciphera, Delmar Pharma, Dnatrix, Ellipses Pharma, Genenta, Genentech/Roche, Imvax, Kintara, Kiyatec, Medicenna Biopharma, Inc., Merck, Merck KGaA, Monteris, Neuvogen, Novartis, Novocure, Oncorus, Oxigene, Regeneron, Stemline, Sumitono Dainippon Pharma, Pyramid Biotherapeutics, Taiho Oncology, Inc., and Y-Mab Therapeutics; grants from Acerta, Epptopoietic Research Corporation, Incyte, Insightec, Omniox, and Tragara; and grants and personal fees from Agenus, Bristol Myers Squibb, Celldex Therapeutics, EMD Serono, and Inovio during the conduct of the study. S. Sahebjam reports other support from Merck, Boehringer Ingelheim, Bristol Myers Squibb, Brooklyn Immunotherapeutics, Eli Lilly, and AbbVie outside the submitted work; in addition, S. Sahebjam has a patent for Systems and Methods for Assessing Patient-Specific Evolution of Resistance to Therapy and Progression of the Disease in Recurrent High-grade Glioma Patients pending. J.H. Sampson reports consulting and licensing fees from Celldex Therapeutics and Medicenna Therapeutics, and is an inventor on patents related to PEP-CMV DC vaccine with tetanus, as well as poliovirus vaccine and D2C7 in the treatment of glioblastoma. In addition, J.H. Sampson reports equity interest in Istari Oncology, which has licensed intellectual property from Duke related to the use of poliovirus and D2C7 in the treatment of glioblastoma, and equity interest in Annias Immunotherapeutics, which has licensed intellectual property from Duke related to the use of the pepCMV vaccine in the treatment of glioblastoma. J. Simes reports grants from Bayer, Roche, Bristol Myers Squibb, AbbVie, AstraZeneca, MSD, and Astellas outside the submitted work. D.A. Berry reports other support from Berry Consultants, LLC outside the submitted work. T.F. Cloughesy reports personal fees from Global Coalition for Adaptive Research, Gan & Lee, Brainstorm, Katmai, Sapience, Inovio, Dnatrix, Tyme, SDP, Novartis, Roche, Kintara, Bayer, Merck, Boehringer Ingelheim, VBL, Amgen, Kiyatec, Odonate, QED, Medfield, Pascal Biosciences, Tocagen, Karyopharm, AbbVie, and VBI outside the submitted work; T.F. Cloughesy also has a patent for 62/819,322 licensed to Katmai Pharmaceuticals and a patent for 63/179,769 licensed to Katmai Pharmaceuticals. In addition, T.F. Cloughesy is cofounder, major stock holder, consultant, and board member of Katmai Pharmaceuticals, is member of the board for the 501c3 Global Coalition for Adaptive Research, holds stock options in Notable Labs and Chimerix and receives milestone payments and possible future royalties, and is member of the scientific advisory board for Break Through Cancer and Cure Brain Cancer Foundation. M.P. Mehta reports personal fees from Karyopharm, Sapience, Zap, Mevion, Xoft, and Tocagen, as well as other support from Oncoceutics and Chimerix outside the submitted work; in addition, M.P. Mehta is NRG Oncology Brain Tumor Committee Chair. M. Weller reports grants and personal fees from Apogenix, Merck (EMD), and Adastra; grants from Quercis; and personal fees from BMS, Medac, MSD, Nerviano, Novartis, Philogen, Orbus, and Ymabs outside the submitted work. A.B. Heimberger reports personal fees from Caris Life Sciences, WCG Oncology Advisory Board, Celldex Therapeutics, Dnatrix, Codiak Biosciences, and Celularity, as well as non-financial support from Carthera and Moleculin during the conduct of the study. M. Khasraw reports grants from BMS, as well as grants and personal fees from AbbVie during the conduct of the study; in addition, M. Khasraw reports personal fees from Roche, Ipsen, Specialized Therapeutics, and Janssen outside the submitted work. No disclosures were reported by the other authors.
Acknowledgments
The authors thank the Society for Neuro-Oncology and their staff for their tremendous contributions to the arrangements and coordination for the Think Tank meeting.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol 2017;18:1373–85. [DOI] [PubMed] [Google Scholar]
- 2. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997–1003. [DOI] [PubMed] [Google Scholar]
- 3. Wen PY, Weller M, Lee EQ, Alexander BA, Barnholtz-Sloan JS, Barthel FP, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-oncol 2020;22:1073–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017;318:2306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chuntova P, Chow F, Watchmaker P, Galvez M, Heimberger AB, Newell EW, et al. Unique challenges for glioblastoma immunotherapy: discussions across neuro-oncology and non-neuro-oncology experts in cancer immunology. Neuro Oncol 2021;23:356–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khasraw M, Reardon DA, Weller M, Sampson JH. PD-1 inhibitors: do they have a future in the treatment of glioblastoma? Clin Cancer Res 2020;26:5287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goswami S, Walle T, Cornish AE, Basu S, Anandhan S, Fernandez I, et al. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat Med 2020;26:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raper D, Louveau A, Kipnis J. How do meningeal lymphatic vessels drain the CNS? Trends Neurosci 2016;39:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 2017;9:eaaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med 2018;24:1459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 2016;19:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev 2008;222:206–21. [DOI] [PubMed] [Google Scholar]
- 13. Mu L, Long Y, Yang C, Jin L, Tao H, Haitao Ge, et al. The IDH1 mutation-induced oncometabolite, 2-hydroxyglutarate, may affect DNA methylation and expression of PD-L1 in gliomas. Front Mol Neurosci 2018;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Röver LK, Gevensleben H, Dietrich J, Bootz F, Landsberg J, Goltz D, et al. PD-1 (PDCD1) promoter methylation is a prognostic factor in patients with diffuse lower-grade gliomas harboring isocitrate dehydrogenase (IDH) mutations. EBioMedicine 2018;28:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee KS, Lee K, Yun S, Moon S, Park Y, Han JH, et al. Prognostic relevance of programmed cell death ligand 1 expression in glioblastoma. J Neurooncol 2018;136:453–61. [DOI] [PubMed] [Google Scholar]
- 16. Strohl WR, Naso M. Bispecific T-cell redirection versus chimeric antigen receptor (CAR)-T cells as approaches to kill cancer cells. Antibodie 2019;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenthal M, Balana C, Linde MEV, Sayehli C, Fiedler WM, Wermke M, et al. Novel anti-EGFRvIII bispecific T cell engager (BiTE) antibody construct in glioblastoma (GBM): Trial in progress of AMG 596 in patients with recurrent or newly diagnosed disease. J Clin Oncol 2019;37:TPS2071–TPS. [Google Scholar]
- 18. Gedeon PC, Schaller TH, Chitneni SK, Choi BD, Kuan CT, Suryadevara CM, et al. A rationally designed fully human EGFRvIII:CD3-targeted bispecific antibody redirects human T cells to treat patient-derived intracerebral malignant glioma. Clin Cancer Res 2018;24:3611–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scott EM, Duffy MR, Freedman JD, Fisher KD, Seymour LW. Solid tumor immunotherapy with T cell engager-armed oncolytic viruses. Macromol Biosci 2018;18. [DOI] [PubMed] [Google Scholar]
- 20. Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res 2014;20:5290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tritz ZP, Ayasoufi K, Malo C, Himes B, Khadka R, Yokanovich L, et al. Combination immunotherapy of αPD-1 and extended half-life IL-2 clears established GL261 gliomas in an MHC class I independent fashion. J Immunol 2020;204:169.15-15. [Google Scholar]
- 22. Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol 2020;6:1003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res 2019;38:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ott M, Tomaszowski KH, Marisetty A, Kong LY, Wei J, Duna M, et al. Profiling of patients with glioma reveals the dominant immunosuppressive axis is refractory to immune function restoration. JCI Insight 2020;5:e134386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao J, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med 2019;25:462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 2019;25:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, López-Janeiro A, Porciuncula A, Idoate MA, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med 2019;25:470–6. [DOI] [PubMed] [Google Scholar]
- 28. Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res 2018;24:4175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol 2019;20:1083–97. [DOI] [PubMed] [Google Scholar]
- 30. Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011;478:197–203. [DOI] [PubMed] [Google Scholar]
- 31. Sadik A, Somarribas Patterson LF, Öztürk S, Mohapatra SR, Panitz V, Secker PF, et al. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 2020;182:1252–70. [DOI] [PubMed] [Google Scholar]
- 32. Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 2010;28:4722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 2016;375:2561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Touat M, Li YY, Boynton AN, Spurr LF, Iorgulescu JB, Bohrson CL, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 2020;580:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khasraw M, Walsh KM, Heimberger AB, Ashley DM. What is the burden of proof for tumor mutational burden in gliomas? Neuro-oncol 2020;23:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Platten M, Bunse L, Wick A, Bunse T, Le Cornet L, Harting I, et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature 2021;592:463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019;565:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu CJ, Schaettler M, Blaha DT, Bowman-Kirigin JA, Kobayashi DK, Livingstone AJ, et al. Treatment of an aggressive orthotopic murine glioblastoma model with combination checkpoint blockade and a multivalent neoantigen vaccine. Neuro-oncol 2020;22:1276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woroniecka K, Fecci PE. Immuno-synergy? Neoantigen vaccines and checkpoint blockade in glioblastoma. Neuro-oncol 2020;22:1233–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019;565:240–5. [DOI] [PubMed] [Google Scholar]
- 41. Choi BD, Yu X, Castano AP, Darr H, Henderson DB, Bouffard AA, et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer 2019;7:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pardridge WM. Blood-brain barrier and delivery of protein and gene therapeutics to brain. Frontiers in Aging Neuroscience 2020;11:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sarkaria JN, Hu LS, Parney IF, Pafundi DH, Brinkmann DH, Laack NN, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-oncol 2018;20:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Portnow J, Wang D, Blanchard MS, Tran V, Alizadeh D, Starr R, et al. Systemic anti–PD-1 immunotherapy results in PD-1 blockade on T cells in the cerebrospinal fluid. JAMA Oncol 2020;6:1947–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi BD, Suryadevara CM, Gedeon PC, Herndon JE 2nd, Sanchez-Perez L, Bigner DD, et al. Intracerebral delivery of a third generation EGFRvIII-specific chimeric antigen receptor is efficacious against human glioma. J Clin Neurosci 2014;21:189–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brady M, Raghavan R, Sampson J. Determinants of intraparenchymal infusion distributions: modeling and analyses of human glioblastoma trials. Pharmaceutics 2020;12:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klinger M, Zugmaier G, Nägele V, Goebeler M-E, Brandl C, Stelljes M, et al. Adhesion of T cells to endothelial cells facilitates blinatumomab-associated neurologic adverse events. Cancer Res 2020;80:91–101. [DOI] [PubMed] [Google Scholar]
- 48. Oved JH, Barrett DM, Teachey DT. Cellular therapy: Immune-related complications. Immunol Rev 2019;290:114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim JE, Patel MA, Mangraviti A, Kim ES, Theodros D, Velarde E, et al. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res 2017;23:124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015;519:366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reap EA, Suryadevara CM, Batich KA, Sanchez-Perez L, Archer GE, Schmittling RJ, et al. Dendritic cells enhance polyfunctionality of adoptively transferred T cells that target cytomegalovirus in glioblastoma. Cancer Res 2018;78:256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mardiana S, Solomon BJ, Darcy PK, Beavis PA. Supercharging adoptive T cell therapy to overcome solid tumor-induced immunosuppression. Sci Transl Med 2019;11:eaaw2293. [DOI] [PubMed] [Google Scholar]
- 54. Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, et al. Focal radiation therapy combined with 4–1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One 2014;9:e101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Do P, Perdue LA, Chyong A, Henry CJ, Porter CC, Dreaden EC. Promoting anti-tumor immunity via bispecific T cell engaging cytokine (biteokine) therapy. J Immunol 2020;204:169.22-22. [Google Scholar]
- 56. Riccione KA, He LZ, Fecci PE, Norberg PK, Suryadevara CM, Swartz A, et al. CD27 stimulation unveils the efficacy of linked class I/II peptide vaccines in poorly immunogenic tumors by orchestrating a coordinated CD4/CD8 T cell response. Oncoimmunology 2018;7:e1502904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Khasraw M, Weller M, Lorente D, Kolibaba K, Lee CK, Gedye C, et al. Bintrafusp alfa (M7824) a bifunctional fusion protein targeting TGF-β and PD-L1: results from a phase 1 expansion cohort in patients with recurrent glioblastoma. Neuro-Oncology Advances 2021;3:vdab058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Choe JH, Watchmaker PB, Simic MS, Gilbert RD, Li AW, Krasnow NA, et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med 2021;13:eabe7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Avanzi MP, Yeku O, Li X, Wijewarnasuriya DP, van Leeuwen DG, Cheung K, et al. Engineered tumor-targeted T cells mediate enhanced anti-tumor efficacy both directly and through activation of the endogenous immune system. Cell Rep 2018;23:2130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Gastineau DA, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro-oncol 2010;12:631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–22. [DOI] [PubMed] [Google Scholar]
- 62. Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget 2017;8:91779–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66. [DOI] [PubMed] [Google Scholar]
- 64. Alexander BM, Ba S, Berger MS, Berry DA, Cavenee WK, Chang SM, et al. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin Cancer Res 2018;24:737–43. [DOI] [PubMed] [Google Scholar]
- 65. Isaacs J, Tan AC, Hanks BA, Wang X, Owzar K, Herndon JE, et al. Clinical trials with biologic primary endpoints in immuno-oncology: concepts and usage. Clin Cancer Res 2021Jul 26 [Epub ahed of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Piantadosi S. Translational clinical trials: an entropy-based approach to sample size. Clinical Trials 2005;2:182–92. [DOI] [PubMed] [Google Scholar]
- 67. Jiang L, Li R, Yan F, Yap TA, Yuan Y. Shotgun: A Bayesian seamless phase I-II design to accelerate the development of targeted therapies and immunotherapy. Contemporary Clinical Trials 2021;104:106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stallard N, Todd S. Seamless phase II/III designs. Stat Methods Med Res 2011;20:623–34. [DOI] [PubMed] [Google Scholar]
- 69. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med 2017;377:62–70. [DOI] [PubMed] [Google Scholar]
- 70. Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 2015;16:e534–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Alexander BM, Trippa L, Gaffey S, IC A-R, Lee EQ, Rinne ML, et al. Individualized Screening Trial of Innovative Glioblastoma Therapy (INSIGhT): a bayesian adaptive platform trial to develop precision medicines for patients with glioblastoma. JCO Precis Oncol 2019;3:PO.18.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wick W, Dettmer S, Berberich A, Kessler T, Karapanagiotou-Schenkel I, Wick A, et al. N2M2 (NOA-20) phase I/II trial of molecularly matched targeted therapies plus radiotherapy in patients with newly diagnosed non-MGMT hypermethylated glioblastoma. Neuro-oncol 2018;21:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pfaff E, Kessler T, Balasubramanian GP, Berberich A, Schrimpf D, Wick A, et al. Feasibility of real-time molecular profiling for patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation—the NCT Neuro Master Match (N2M2) pilot study. Neuro-oncol 2017;20:826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Prowell TM, Theoret MR, Pazdur R. Seamless oncology-drug development. N Engl J Med 2016;374:2001. [DOI] [PubMed] [Google Scholar]
- 75. Murthy H, Iqbal M, Chavez JC, Kharfan-Dabaja MA. Cytokine release syndrome: current perspectives. Immunotargets Ther 2019;8:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thall PF, Millikan RE, Mueller P, Lee SJ. Dose-finding with two agents in phase I oncology trials. Biometrics 2003;59:487–96. [DOI] [PubMed] [Google Scholar]
- 77. Yin G, Li Y, Ji Y. Bayesian dose-finding in phase I/II clinical trials using toxicity and efficacy odds ratios. Biometrics 2006;62:777–87. [DOI] [PubMed] [Google Scholar]
- 78. Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 2009;101:708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rahma OE, Reuss JE, Giobbie-Hurder A, Shoja ERG, Abu-Shawer O, Mehra P, et al. Early 3+3 trial dose-escalation phase I clinical trial design and suitability for immune checkpoint inhibitors. Clin Cancer Res 2021;27:485–91. [DOI] [PubMed] [Google Scholar]
- 80. Wen PY, Chang SM, MJVd B, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol 2017;35:2439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 2017;14:307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weathers SP, Penas-Prado M, Pei BL, Ling X, Kassab C, Banerjee P, et al. Glioblastoma-mediated immune dysfunction limits CMV-specific T cells and therapeutic responses: results from a phase I/II trial. Clin Cancer Res 2020;26:3565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cerignoli F, Abassi YA, Lamarche BJ, Guenther G, Santa Ana D, Guimet D, et al. In vitro immunotherapy potency assays using real-time cell analysis. PLoS One 2018;13:e0193498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yuan Y, Lee JJ, Hilsenbeck SG. Model-assisted designs for early-phase clinical trials: simplicity meets superiority. JCO Prec Oncol 2019;3:PO.19.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yuan Y, Yin G. Bayesian phase I/II adaptively randomized oncology trials with combined drugs. Ann Appl Stat 2011;5:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Macchia I, Urbani F, Proietti E. Immune monitoring in cancer vaccine clinical trials: critical issues of functional flow cytometry-based assays. Biomed Res Int 2013;2013:726239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rao G, Latha K, Ott M, Sabbagh A, Marisetty A, Ling X, et al. Anti-PD-1 induces M1 polarization in the glioma microenvironment and exerts therapeutic efficacy in the absence of CD8 cytotoxic T cells. Clin Cancer Res 2020;26:4699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. PNAS 2004;101:10691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. Programmed death-1 pathway limits central nervous system inflammation and neurologic deficits in murine experimental stroke. Stroke 2011;42:2578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]