FIGURE 1.
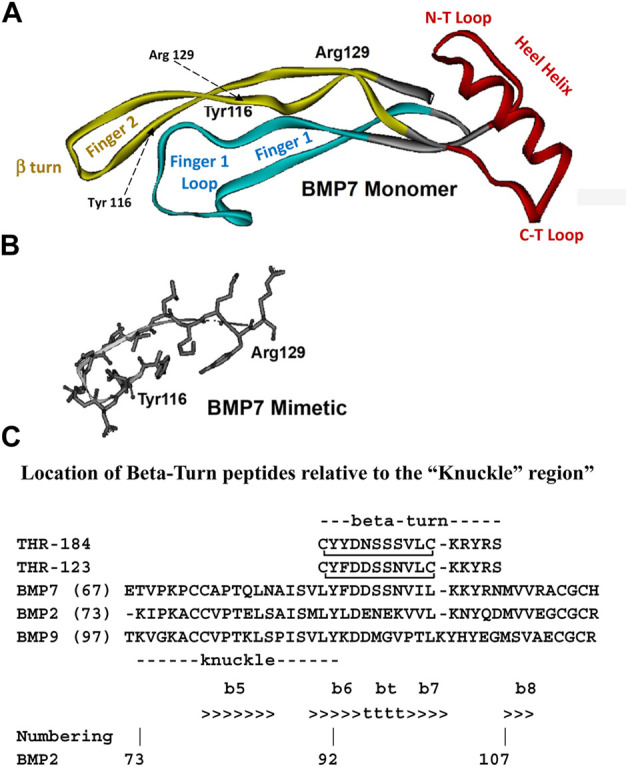
Structure diagrams of the BMP-7 monomer and the region covered by the mimetic. (A) A ribbon diagram showing the secondary structure of the BMP monomer, which contains three structural regions: antiparallel beta sheets of “Finger 1” (with the large terminal loop), “Finger 2” (with the tight beta-turn), and the “Heel” alpha helix. Initial targets for mimetic development were the terminal loops of fingers 1 and 2, loops at the C-terminal, and N-terminal loops at the ends of the Heel helix. (B) The region around the beta turn of Finger two proved to have activity similar to BMP-7 and became the lead for further mimetic development. (C) The “beta-turn” region covered by the mimetic is immediately C-terminal to the “knuckle” region covered by other BMP mimetics. Residue position numbers are based on BMP-2 residue numbers. Secondary structure: beta sheet (>>>>), segments of which are labelled e.g., “b6”; beta turn (bt, tttt). Peptide disulfide bond: C_C.
