Figure 3.
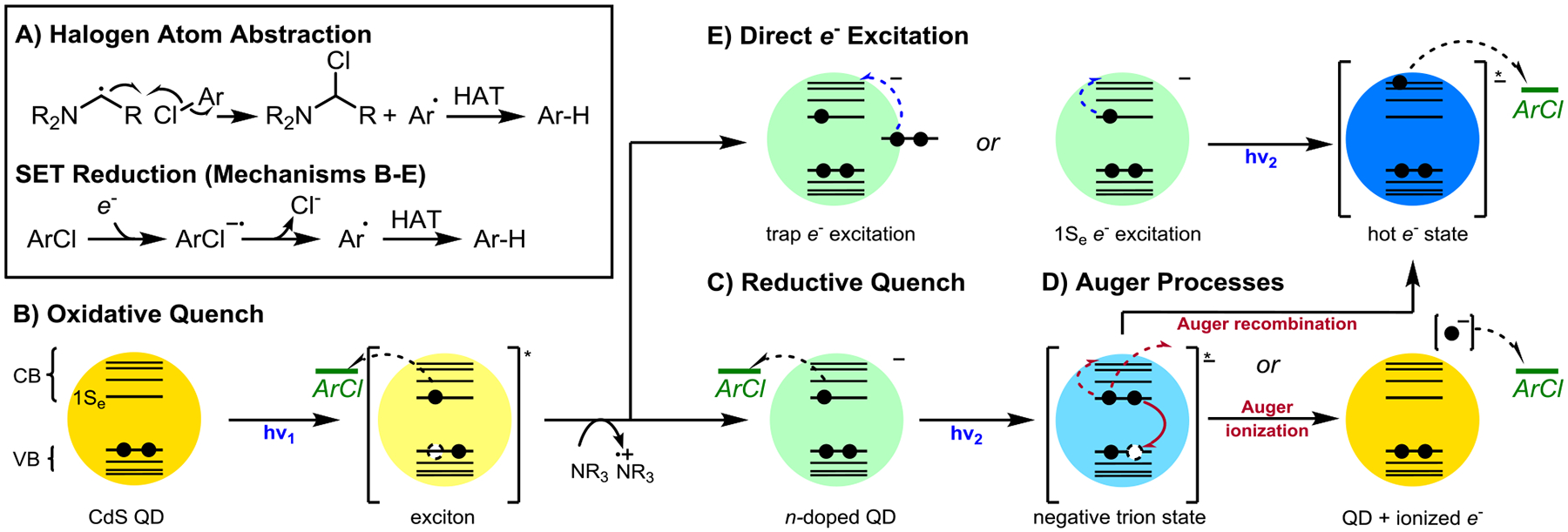
Mechanistic possibilities for aryl chloride reduction. A: Halogen atom abstraction by organic radicals derived from the reductant. B: Reduction of substrate by a neutral excited QD. C: Reduction of substrate by a ground-state anionic QD after reductive quenching. D: Reduction of substrate by a hot or ionized electron generated via Auger processes of a negative trion state. E: Reduction of substrate by a hot electron generated via direct excitation of electrons in the 1Se state or surface trap states.
