Figure 15.
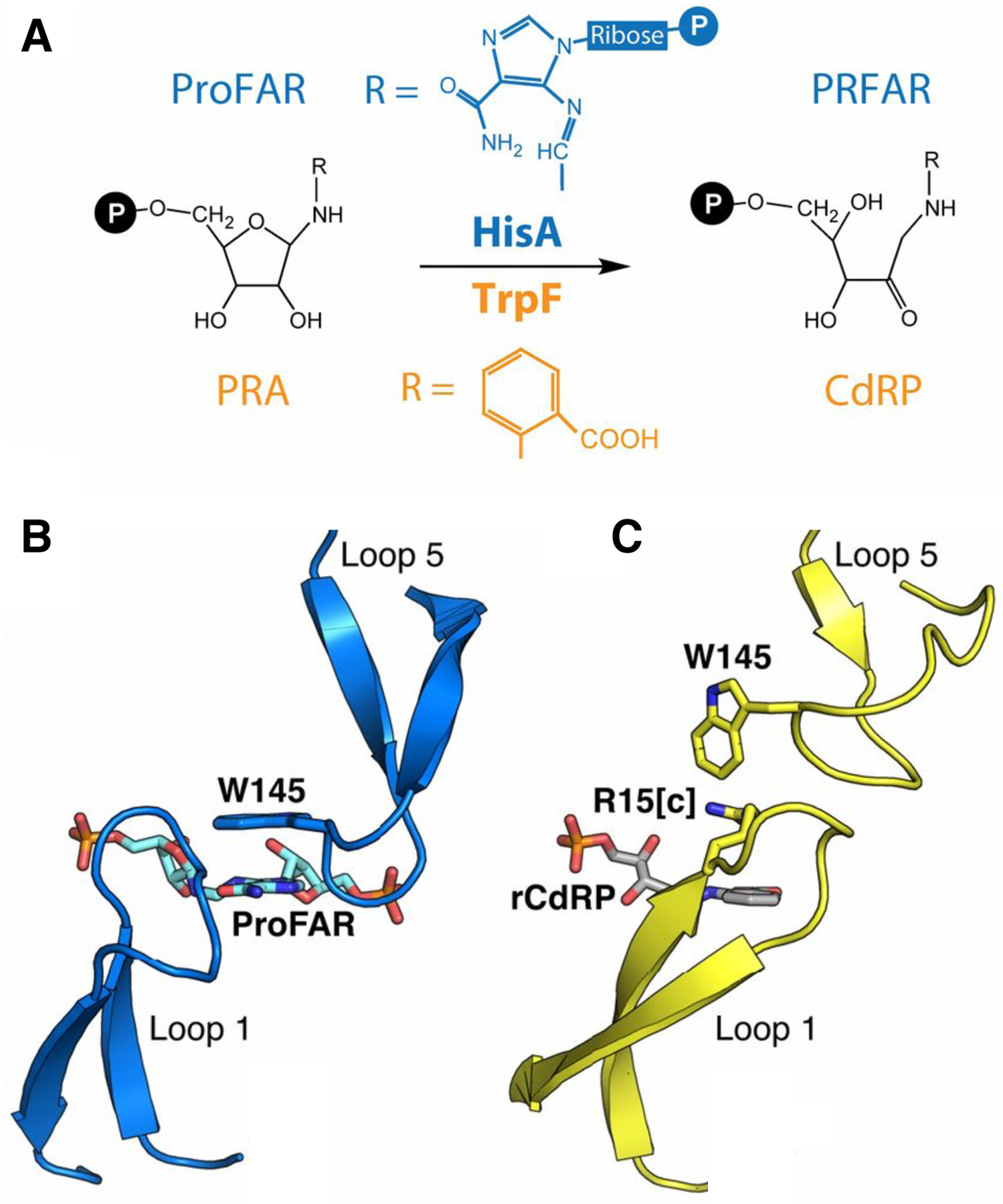
A) HisA and TrpF catalyze Amadori rearrangements of structurally different substrates. B) The ProFAR substrate in the active site of the catalytically inactive D7N D176A HisA (PDB 5A5W). C) A TrpF product analog, rCdRP (reduced 1′-(2′-carboxyphenylamino)-1′-deoxyribulose 5′-phosphate), positioned in the active site of HisA(D7N/dup13–15/D10G) based upon its position in the active site of the ortholog PriA (PDB 2Y85). Reprinted with permission from Proc Natl Acad Sci USA 114(18):4727–32, 2017. Structural and functional innovations in the real-time evolution of new (betaalpha)8 barrel enzymes. Newton MS, Guo X, Soderholm A, Nasvall J, Lundstrom P, Andersson DI, et al.
