Figure 17.
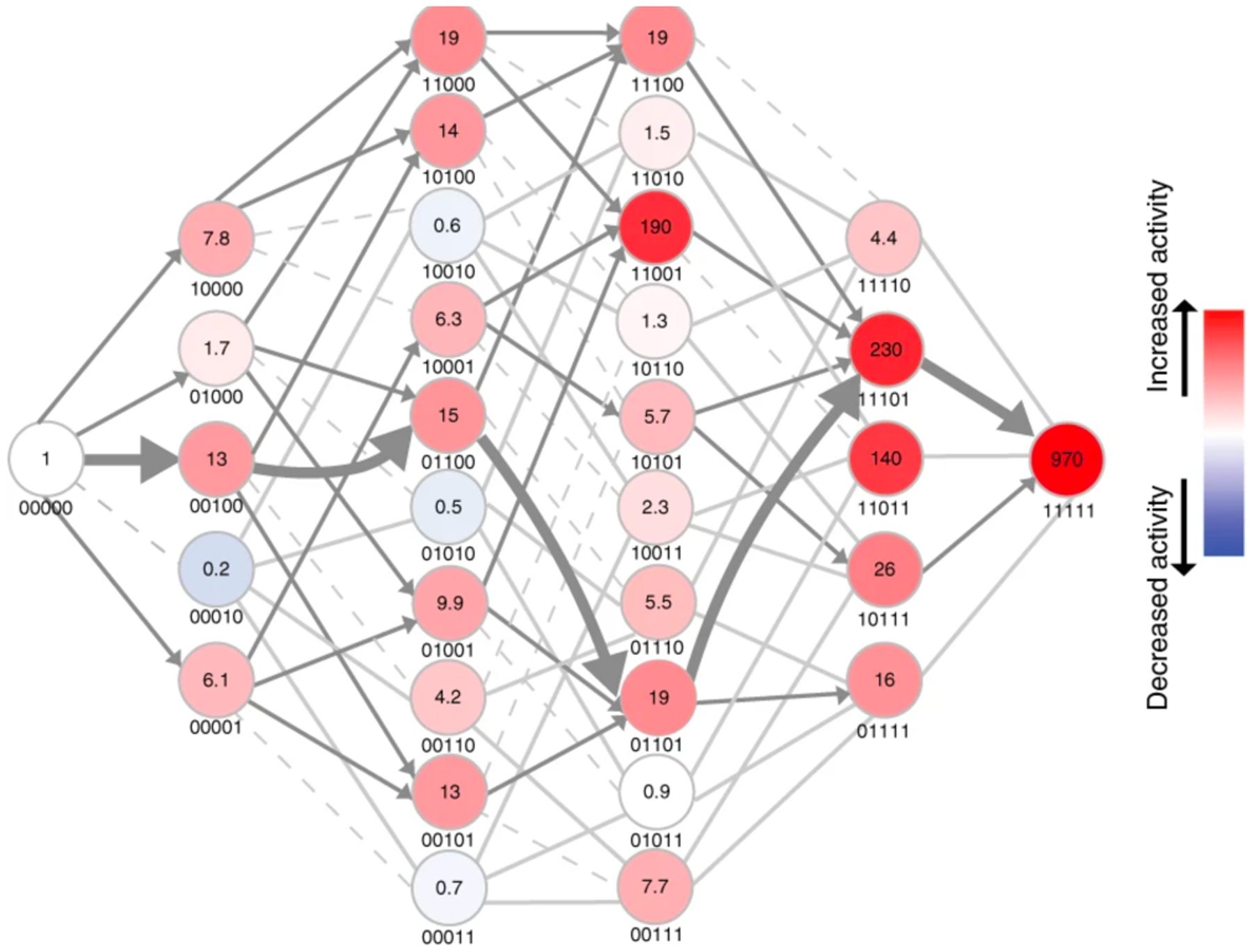
The adaptive landscape between methyl parathion hydrolase (right) and an ancestor in which five key residues had been reverted to the ancestral state (left). The numbers under each node indicate the absence (0) or presence (1) of the derived residue at positions 73, 193, 258, 271 and 273. Colors indicate the mean methyl parathion hydrolase activity in lysates for three biological replicates. Dashed light grey lines indicate paths that are evolutionarily inaccessible because they go through an intermediate with decreased activity. Grey arrows indicate steps in which activity is increased. Reprinted by permission from Springer Nature: Nat Chem Biol. 15(11):1120–8, 2019. Higher-order epistasis shapes the fitness landscape of a xenobiotic-degrading enzyme, Yang G, Anderson DW, Baier F, Dohmen E, Hong N, Carr PD, et al. Copyright 2019.
