Figure 18.
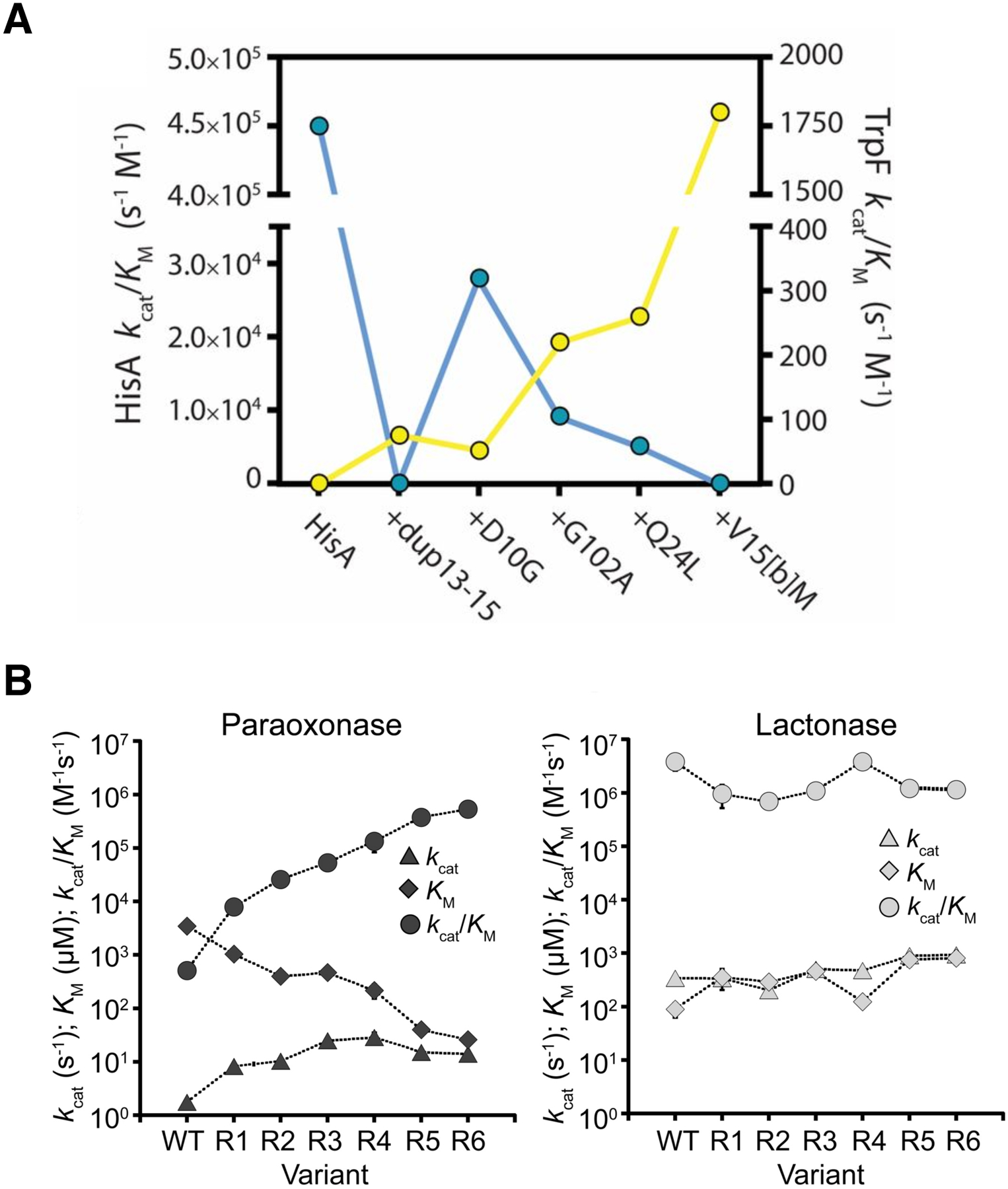
Tradeoffs between the original and new activities of an evolving enzyme. A) A strong tradeoff between TrpF (yellow) and HisA (blue) activities in HisA. An allele encoding HisA(dup13–15/D10G) evolved into a specialist TrpF in S. enterica in which this bifunctional enzyme supported synthesis of both histidine and tryptophan, but poorly. Reprinted with permission from Proc Natl Acad Sci USA 114(18):4727–32, 2017. Newton MS, Guo X, Soderholm A, Nasvall J, Lundstrom P, Andersson DI, et al. Structural and functional innovations in the real-time evolution of new (betaalpha)8 barrel enzymes. B) A weak tradeoff between the original homoserine lactonase activity and the promiscuous paraoxonase activity of AiiA through six rounds of directed evolution. Reprinted with permission from Biochemistry 55:4583–4593, 2016. Yang G, Hong N, Baier F, Jackson CJ, Tokuriki N. Conformational tinkering drives evolution of a promiscuous activity through indirect mutational effects. Copyright 2016 American Chemical Society.
