Figure 1.
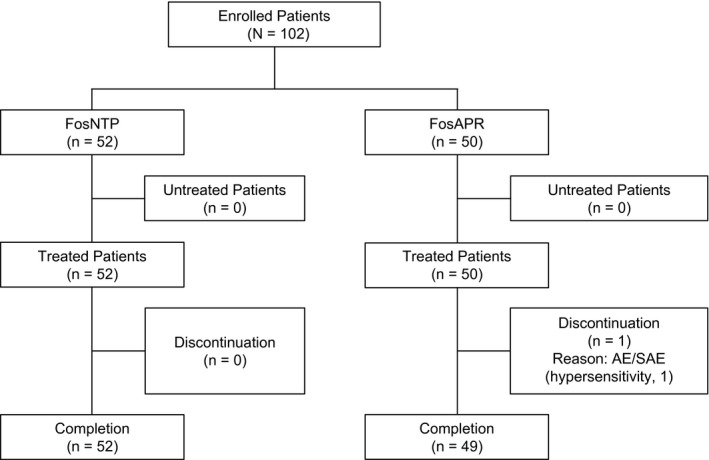
Consolidated Standards of Reporting Trials diagram showing the patient disposition. AE indicates adverse event; FosAPR, fosaprepitant; FosNTP, fosnetupitant; SAE, serious adverse event.

Consolidated Standards of Reporting Trials diagram showing the patient disposition. AE indicates adverse event; FosAPR, fosaprepitant; FosNTP, fosnetupitant; SAE, serious adverse event.