FIGURE 4.
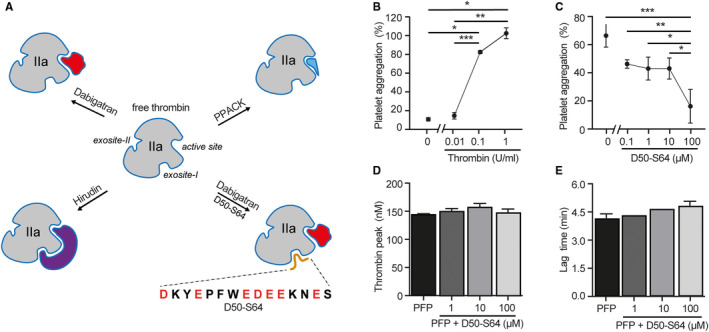
Thrombin binding capacity of peptide D50‐S64. A, Schematic illustration of free thrombin with its active site and exosite‐I and ‐II and in complex with different direct thrombin inhibitors. Dabigatran is a non‐peptidic, reversible inhibitor interacting with thrombin’s active site only, leaving exosite‐I and ‐II available. Hirudin binding is irreversible and will interact with both the active site and exosite‐I. PPACK is a peptide derivative, which forms an irreversible interaction with the active site, leaving exosite‐I available. Peptide D50‐S64 was designed, which mimics the PAR‐1 sequence that will interact with exosite‐I of thrombin. Incubation of thrombin with both peptide and dabigatran will reflect the situation of hirudin‐blocked thrombin. B, Light transmission aggregometry was performed with purified platelets (200.106/ml) incubated with thrombin (0–1 U/ml). C, Thrombin‐induced (0.1 U/ml) platelet aggregation was dose‐dependently inhibited with peptide D50‐S64 (0–100 µM). Individual points of three separate experiments with three different platelet donors are shown. A standardized CAT assay was performed using pooled PFP, to which purified TF and phospholipids were added. A representative thrombin generation curve of PFP ± a dose response of peptide D50‐S64 (0–100 μM) is shown. The peptide did not affect (D) peak height (nM) or (E) lag time (min) of thrombin generation. Mean ± standard deviation were compared using one‐way analysis of variance with Tukey’s multiple comparisons test. *P < .05, **P < .01, ***P < .001. CAT, calibrated automated thrombogram; PAR‐1, protease‐activated receptor‐1 ; PFP, platelet‐free plasma; TF, tissue factor
