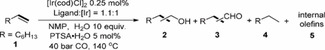
Table 2.
Ir‐catalyzed hydroformylation/reduction of oct‐1‐ene: Ligand variation.[a]
|
| |||||
|---|---|---|---|---|---|
|
|
Ligand |
Yield [%][b] |
|||
|
2 (n:iso)[c] |
3 (n:iso) |
4 |
5 |
||
|
1[d] |
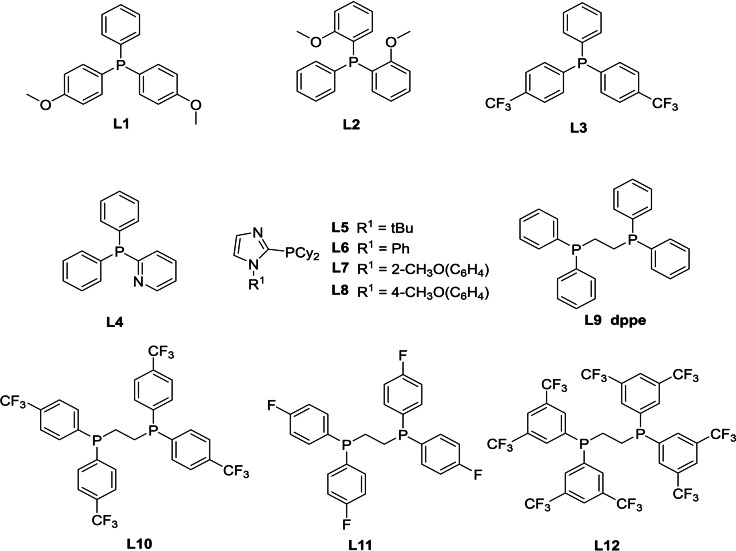
PPh3 |
n.d. |
81 (67 : 33) |
19 |
<1 |
|
2[e] |
PPh3 |
n.d. |
92 (71 : 29) |
8 |
<1 |
|
3[f,g] |
PPh3 |
n.d. |
94 (70 : 30) |
5 |
<1 |
|
4[f] |
P(p‐CH3OC6H4)3 |
n.d. |
91 (74 : 26) |
6 |
3 |
|
5[f] |
L1 |
n.d. |
96 (72 : 28) |
2 |
1 |
|
6[f] |
L2 |
n.d. |
81 (65 : 35) |
9 |
8 |
|
7[f] |
L3 |
n.d. |
89 (50 : 50) |
9 |
1 |
|
8[f] |
Ph2PPy (L4) |
n.d. |
88 (64 : 36) |
11 |
<1 |
|
9[f,g] |
PCy3 |
n.d. |
94 (75 : 25) |
6 |
<1 |
|
10 |
L5 |
n.d. |
74 (65 : 35) |
17 |
8 |
|
11 |
L6 |
n.d. |
72 (67 : 33) |
18 |
9 |
|
12 |
L7 |
n.d. |
79 (64 : 36) |
14 |
7 |
|
13 |
L8 |
n.d. |
84 (67 : 33) |
10 |
5 |
|
14 |
DPPE (L9) |
1 |
47 (58 : 42) |
36 |
15 |
|
15 |
L10 |
47 (66 : 33) |
26 (46 : 54) |
18 |
3 |
|
16[h] |
L10 |
62 (63 : 37) |
16 (32 : 68) |
19 |
3 |
|
17 |
L11 |
21 (57 : 43) |
43 (46 : 54) |
34 |
2 |
|
18 |
L12 |
17 (50 : 50) |
44 (44 : 56) |
26 |
13 |
[a] Rection conditions: 1.0 mmol of oct‐1‐ene, 0.25 mol % of [Ir(cod)Cl]2 (0.5 mol % of [Ir]), 0.55 mol % of ligand (ligand:[Ir]=1.1 : 1), NMP 1.0 mL, CO 40 bar, 10 equiv. of H2O, 5 mol % of PTSA⋅H2O, 140 °C, 20 h. [b] Determined by GC using isooctane (57 mg) as internal standard. [c] n:iso is the ratio of linear product to all branched products. [d] Synthesis gas 40 bar (CO:H2=1 : 1) and without acid. [e] Synthesis gas 40 bar (CO:H2=3 : 1) and without acid. [f] 1.1 mol % of ligand (ligand:[Ir]=2.2 : 1). [g] 48 h. [h] 1 mol % of [Ir] and 1.1 mol of L10.