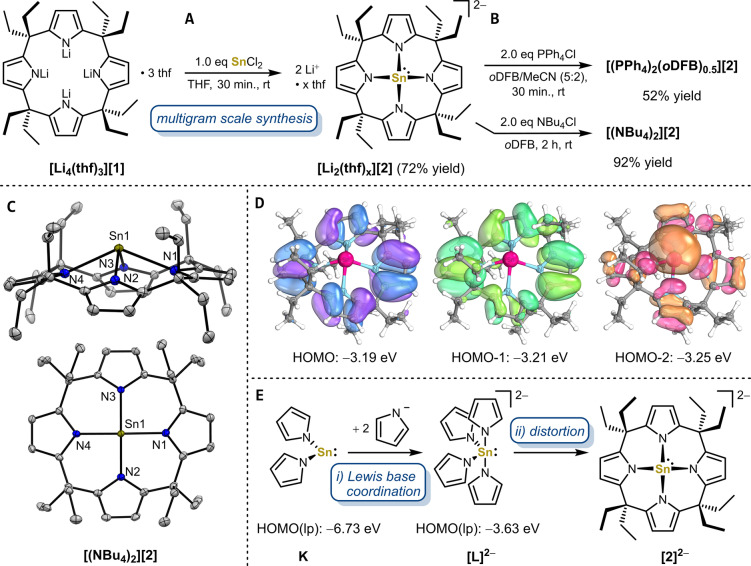
Figure 2.
A) Synthesis of the dianionic calix[4]pyrrolato stannate(II) as the lithium salt [Li2(thf) x ][2] and B) subsequent salt exchange reactions with PPh4Cl and NBu4Cl forming the phosphonium salt [(PPh4)2(oDFB)0.5][2] and the ammonium salt [(NBu4)2][2]. C) Solid‐state molecular structure of [(NBu4)2][2]. Displacement ellipsoids are drawn at 50 % probability level. Hydrogens and NBu4 + counter cations are omitted for clarity. Selected bond lengths [pm] and angels [°]: Sn1−N1 232.27(16), Sn1−N2 230.61(15), Sn1−N3 230.73(15), Sn1−N4 232.11(15), Sn1−N4‐plane 95.8, N1−Sn1−N4 133.23(5), N2−Sn1−N3 129.10(5), cis−N−Sn−N between 79.64(5) and 80.85(5). D) Occupied frontier molecular orbitals calculated at the B97M‐D3(BJ)/def2‐TZVPP CPCM(THF)//B97M‐D3(BJ)/def2‐TZVPP level of theory. E) Effects of Lewis base coordination (i) and distortion into the calix[4]pyrrole coordination geometry (ii) on the energy of the lone pair (lp) containing orbital at tin(II) for the hypothetical bis(pyrrolato)stannylene K, tetrakis(pyrrolato)stannate(II) [L]2− , and [2]2− .