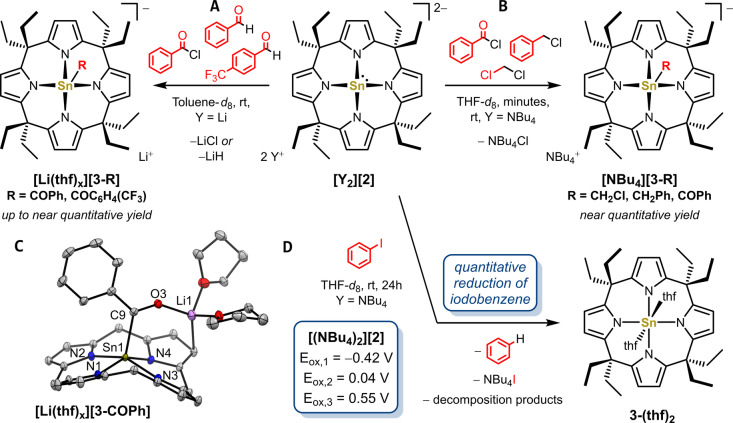
Figure 5.
Reactivity of A) [Li2(thf) x ][2] and B) [(NBu4)2][2] with electrophiles and D) reaction of [(NBu4)2][2] with iodobenzene. Yields where determined by NMR spectroscopy. Oxidation potentials were determined by cyclic voltammetry of [(NBu4)2][2] (10−3 M) and Bu4NPF6 (0.01 M) in 1,2‐difluorobenzene at a scan rate of 0.05 V s−1. C) Solid state molecular structure of [Li(thf) x ][3‐COPh]. Displacement ellipsoids are drawn at 50 % probability level. Hydrogens and toluene solvent molecules are omitted for clarity. Selected bond lengths [pm] and angles [°]: Sn1−N1 208.11(10), Sn1−N2 210.25(10), Sn1−N3 211.02(12), Sn1−N4 211.31(11), Sn1−N4‐plane 49.4, Sn1−C9 220.94(13), C9−O3 123.20(14), N1−Sn1−N4 152.56(4), N2−Sn1−N3 153.09(4), cis−N−Sn−N between 84.37(4) and 89.43(4).