Figure 1. The N-glycosylation status of various Campylobacter isolates.
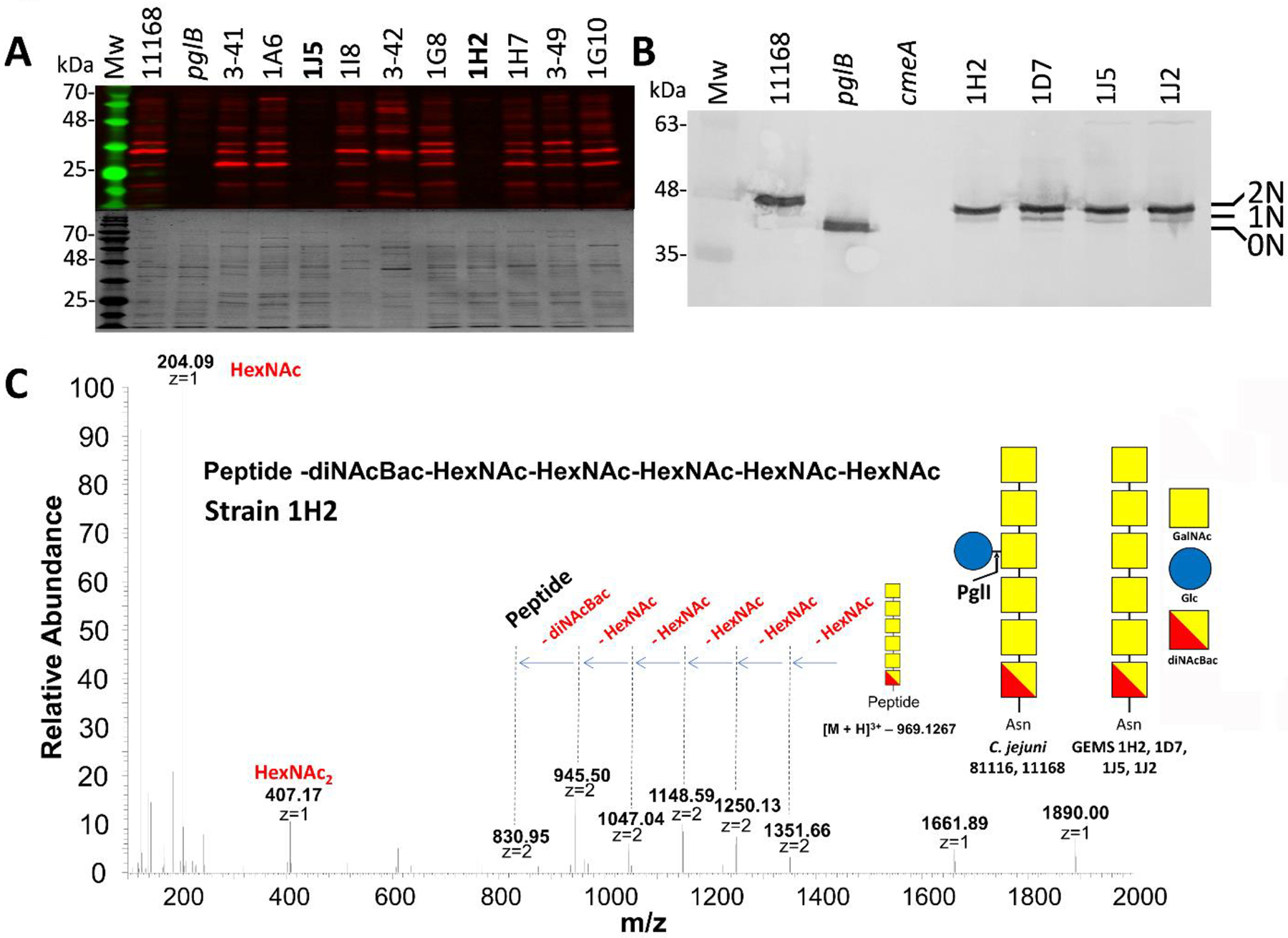
(A) Western blot (upper panel) with Cj-N-glycan specific R1 antiserum of equal amounts (3 μg) of whole cell lysates of C. jejuni WT (NCTC 11168), the isogenic pglB mutant (pglB) and other C. jejuni and C. coli isolates. Coomassie blue-stained gels were run in parallel as loading controls (lower panel). Western blots and Coomassie blue-stained gels for all the other GEMS isolates are provided in Figure S1. The full list of GEMS strains is provided in Table S3. (B) Western blot with Cj-CmeA-specific antiserum of C. jejuni WT (NCTC 11168), the isogenic pglB mutant (pglB), the Cj-cmeA mutant (cmeA, background control) and the GEMS strains (as indicated) that did not react with R1 serum. Non-, mono- and di-glycosylated CmeA proteins are indicated as 0N, 1N and 2N, respectively. Note that mono-glycosylated CmeA cannot be detected in the Cj-WT since it produces only di-glycosylated (2N) CmeA while the N-glycan is absent in the pglB mutant (0N). Molecular weight markers (Mw in kDa) are indicated on the left. A full top-to-bottom scan is provided in Figure S2. (C) Liquid chromatography-mass spectrometry (LC-MS)-based analyses of CmeA glycopeptides. HCD MS2 spectrum showing the fragmentation pattern of the carbohydrate portion on the CmeA peptide resulted in the identification of a HexNAc5-diNAcBac N-glycan attached to CmeA of C. jejuni 1H2. Spectra for N-glycopeptides from strains 1J2, 1J5 and 1D7 are provided in Figure S3. Since the genome and/or proteome of the GEMS strains was not available for databank searches, only the attached glycan structure was sequenced. The symbolic representation of the glucose-containing N-glycan from C. jejuni strain NCTC11168 is depicted for comparison; analysis of the full-length C. jejuni heptasaccharide N-glycan (HexNAc-HexNAc-HexNAc(Hex)-HexNAc-HexNAc-diNAcBac) attached to CmeA of 11168 WT is in Figure S3.
