Abstract
Background
Idiopathic sudden sensorineural hearing loss (ISSNHL) is common, and defined as a sudden decrease in sensorineural hearing sensitivity of unknown aetiology. Systemic corticosteroids are widely used, however their value remains unclear. Intratympanic injections of corticosteroids have become increasingly common in the treatment of ISSNHL.
Objectives
To assess the effects of intratympanic corticosteroids in people with ISSNHL.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Trials Register; CENTRAL (2021, Issue 9); PubMed; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials (search date 23 September 2021).
Selection criteria
We included randomised controlled trials (RCTs) involving people with ISSNHL and follow‐up of over a week. Intratympanic corticosteroids were given as primary or secondary treatment (after failure of systemic therapy).
Data collection and analysis
We used standard Cochrane methods, including GRADE to assess the certainty of the evidence. Our primary outcome was change in hearing threshold with pure tone audiometry. Secondary outcomes included the proportion of people whose hearing improved, final hearing threshold, speech audiometry, frequency‐specific hearing changes and adverse effects.
Main results
We included 30 studies, comprising 2133 analysed participants. Some studies had more than two treatment arms and were therefore relevant to several comparisons. Studies investigated intratympanic corticosteroids as either primary (initial) therapy or secondary (rescue) therapy after failure of initial treatment.
1. Intratympanic corticosteroids versus systemic corticosteroids as primary therapy
We identified 16 studies (1108 participants). Intratympanic therapy may result in little to no improvement in the change in hearing threshold (mean difference (MD) ‐5.93 dB better, 95% confidence interval (CI) ‐7.61 to ‐4.26; 10 studies; 701 participants; low‐certainty). We found little to no difference in the proportion of participants whose hearing was improved (risk ratio (RR) 1.04, 95% CI 0.97 to 1.12; 14 studies; 972 participants; moderate‐certainty). Intratympanic therapy may result in little to no difference in the final hearing threshold (MD ‐3.31 dB, 95% CI ‐6.16 to ‐0.47; 7 studies; 516 participants; low‐certainty). Intratympanic therapy may increase the number of people who experience vertigo or dizziness (RR 2.53, 95% CI 1.41 to 4.54; 1 study; 250 participants; low‐certainty) and probably increases the number of people with ear pain (RR 15.68, 95% CI 6.22 to 39.49; 2 studies; 289 participants; moderate‐certainty). It also resulted in persistent tympanic membrane perforation (range 0% to 3.9%; 3 studies; 359 participants; very low‐certainty), vertigo/dizziness at the time of injection (1% to 21%, 3 studies; 197 participants; very low‐certainty) and ear pain at the time of injection (10.5% to 27.1%; 2 studies; 289 participants; low‐certainty).
2. Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as primary therapy
We identified 10 studies (788 participants). Combined therapy may have a small effect on the change in hearing threshold (MD ‐8.55 dB better, 95% CI ‐12.48 to ‐4.61; 6 studies; 435 participants; low‐certainty). The evidence is very uncertain as to whether combined therapy changes the proportion of participants whose hearing is improved (RR 1.27, 95% CI 1.15 to 1.41; 10 studies; 788 participants; very low‐certainty). Combined therapy may result in slightly lower (more favourable) final hearing thresholds but the evidence is very uncertain, and it is not clear whether the change would be important to patients (MD ‐9.11 dB, 95% CI ‐16.56 to ‐1.67; 3 studies; 194 participants; very low‐certainty). Some adverse effects only occurred in those who received combined therapy. These included persistent tympanic membrane perforation (range 0% to 5.5%; 5 studies; 474 participants; very low‐certainty), vertigo or dizziness at the time of injection (range 0% to 8.1%; 4 studies; 341 participants; very low‐certainty) and ear pain at the time of injection (13.5%; 1 study; 73 participants; very low‐certainty).
3. Intratympanic corticosteroids versus no treatment or placebo as secondary therapy
We identified seven studies (279 participants). Intratympanic therapy may have a small effect on the change in hearing threshold (MD ‐9.07 dB better, 95% CI ‐11.47 to ‐6.66; 7 studies; 280 participants; low‐certainty). Intratympanic therapy may result in a much higher proportion of participants whose hearing is improved (RR 5.55, 95% CI 2.89 to 10.68; 6 studies; 232 participants; low‐certainty). Intratympanic therapy may result in lower (more favourable) final hearing thresholds (MD ‐11.09 dB, 95% CI ‐17.46 to ‐4.72; 5 studies; 203 participants; low‐certainty). Some adverse effects only occurred in those who received intratympanic injection. These included persistent tympanic membrane perforation (range 0% to 4.2%; 5 studies; 185 participants; very low‐certainty), vertigo or dizziness at the time of injection (range 6.7% to 33%; 3 studies; 128 participants; very low‐certainty) and ear pain at the time of injection (0%; 1 study; 44 participants; very low‐certainty).
4. Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as secondary therapy
We identified one study with 76 participants. Change in hearing threshold was not reported. Combined therapy may result in a higher proportion with hearing improvement, but the evidence is very uncertain (RR 2.24, 95% CI 1.10 to 4.55; very low‐certainty). Adverse effects were poorly reported with only data for persistent tympanic membrane perforation (rate 8.1%, very low‐certainty).
Authors' conclusions
Most of the evidence in this review is low‐ or very low‐certainty, therefore it is likely that further studies may change our conclusions.
For primary therapy, intratympanic corticosteroids may have little or no effect compared with systemic corticosteroids. There may be a slight benefit from combined treatment when compared with systemic treatment alone, but the evidence is uncertain.
For secondary therapy, there is low‐certainty evidence that intratympanic corticosteroids, when compared to no treatment or placebo, may result in a much higher proportion of participants whose hearing is improved, but may only have a small effect on the change in hearing threshold. It is very uncertain whether there is additional benefit from combined treatment over systemic steroids alone.
Although adverse effects were poorly reported, the different risk profiles of intratympanic treatment (including tympanic membrane perforation, pain and dizziness/vertigo) and systemic treatment (for example, blood glucose problems) should be considered when selecting appropriate treatment.
Keywords: Humans; Adrenal Cortex Hormones; Adrenal Cortex Hormones/adverse effects; Dizziness; Hearing Loss, Sensorineural; Hearing Loss, Sensorineural/drug therapy; Pain; Pain/drug therapy; Tympanic Membrane Perforation; Tympanic Membrane Perforation/drug therapy; Vertigo; Vertigo/drug therapy
Plain language summary
Treatment of sudden hearing loss with corticosteroids applied into the middle ear
What is sudden hearing loss?
Sudden hearing loss is a condition characterised by the sudden onset (usually within 72 hours) of reduced or absent hearing.
How is it treated?
People have often used corticosteroids – a type of anti‐inflammatory medicine ‐ to treat the condition. These medicines are usually taken by mouth or injected into the body (known as systemic corticosteroids), but can also be given as an injection directly into the middle ear, through the eardrum (known as intratympanic corticosteroids).
What did we want to find out?
It is not clear whether intratympanic treatment with corticosteroids is effective, or which of these treatments (intratympanic or systemic) is best for treating this condition.
What did we do?
We searched for all relevant studies in the medical literature, compared the results and summarised the evidence. We also assessed how certain the evidence was, considering factors such as study size and the way studies were conducted. Based on our assessments, we categorised the evidence as being of very low, low, moderate or high certainty.
What did we find?
We found 30 studies that included 2133 people. These studies compared intratympanic treatment with corticosteroids with no treatment, with placebo (sham or dummy treatment) and with corticosteroids that were taken by mouth or injection into the body (systemic corticosteroids). We took into account whether people were having their first treatment for sudden deafness or whether they had previously had some other kind of treatment (which had not worked).
For people having their first treatment for sudden deafness
We did not find any studies that compared intratympanic corticosteroids to no treatment or placebo (dummy) treatment.
Intratympanic corticosteroids might result in little or no difference in hearing when compared to people who receive systemic corticosteroids, and might make little to no difference in the number of people whose hearing improves. The side effects may be different with these two types of treatment. With intratympanic treatment, people may have an increase in the risk of dizziness or ear pain as compared to systemic corticosteroids, typically at the time of injection, and some may develop a small hole in the ear drum. However, systemic treatment may also cause an increased risk of different side effects, such as problems with sugar levels in the blood.
Taking intratympanic corticosteroids as well as systemic corticosteroids might result in a small improvement in hearing compared to systemic corticosteroids alone, but it is uncertain how many people would notice an improvement. As above, intratympanic treatment may cause some side effects, but we cannot be certain of the number of people who may experience these.
For people having additional treatment for sudden deafness (when their first treatment did not work)
When compared to no treatment or a placebo (dummy) treatment, intratympanic corticosteroids may result in a much larger number of people having an improvement in their hearing but may only improve hearing slightly. As with first treatment, intratympanic injections might cause some side effects, such as pain or dizziness at the time of the injection, or development of a small hole in the ear drum. We are not certain how often these side effects will happen.
We are very uncertain whether adding intratympanic treatment to systemic treatment will result in an improvement in hearing.
What are the limitations of the evidence?
We considered most of the evidence we found to be of low or very low certainty. This was because there were often some problems with how the studies had been carried out, there may have been few people included in the studies and sometimes results from different studies were conflicting. Therefore, the conclusions of this review may change as new studies are published.
How up‐to‐date is this evidence?
The evidence in this Cochrane Review is current to 23 September 2021.
Summary of findings
Summary of findings 1. Intratympanic corticosteroids versus systemic corticosteroids as primary therapy.
| Intratympanic corticosteroids versus systemic corticosteroids as primary therapy | ||||||
|
Patient or population: sudden sensorineural hearing loss Settings: initial therapy Intervention: intratympanic steroid therapy Comparison: systemic steroid therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | No of participants (studies) | Relative effect (95% CI) | Certainty of the evidence (GRADE) | Comments | |
|
Systemic therapy (assumed risk) |
Intratympanic therapy (corresponding risk) |
|||||
| Change in hearing threshold determined by PTA Range 0 dB to 140 dB Negative values represent lowering and positive values represent raising of the hearing threshold. A lower hearing threshold represents hearing improvement). |
The mean change in PTA ranged across control groups from ‐30.07 dB to ‐15.1 dB | The mean change in PTA in the intervention groups was on average ‐5.93 dB greater (from ‐4.26 greater to ‐7.61 greater) | 701 (10 studies) |
MD ‐5.93 dB (95% CI ‐7.61 to ‐4.26) |
⊕⊕⊝⊝ low1 | Intratympanic therapy may have a trivial/no effect on the change in hearing threshold when compared to systemic steroids (as primary therapy). |
| Proportion of patients whose hearing is improved | 731 per 1000a | 760 per 1000 (709 to 818) | 972 (14 studies) |
RR 1.04 (95% CI 0.97 to 1.12) |
⊕⊕⊕⊝ moderate2 | Intratympanic therapy probably results in little to no difference in the proportion of patients whose hearing is improved compared to systemic corticosteroids (as primary therapy). |
| Final hearing threshold determined by PTA (a lower value represents better hearing) | The mean final PTA ranged across control groups from 25.1 dB to 59 dB | The mean final PTA in the intervention groups was on average ‐3.31 dB lower (‐6.16 lower to ‐0.47 lower) | 516 (7 studies) |
MD ‐3.31 dB (95% CI ‐6.16 to ‐0.47) |
⊕⊕⊝⊝ low3 |
Intratympanic therapy may result in little to no difference in the final hearing threshold (as primary therapy). |
| Adverse eventsb | Events in control group | Events in intervention group | No of participants (studies) | Relative effect (95% CI) |
Certainty of the evidence (GRADE) |
Comments |
| Tympanic membrane perforation | Comparison not applicablec | Ranged from 0% (0/30) to 3.9% (5/129) | 463 (4 studies) | Not calculable | ⊕⊝⊝⊝ very low4 |
The evidence is very uncertain regarding the risk of tympanic membrane perforation for those who received intratympanic corticosteroid as primary treatment. |
| Vertigo/dizziness: timing not reportedd | 13/121 (10.7%) | 35/129 (27.1%) | 250 (1 study) | RR 2.53 (1.41 to 4.54) | ⊕⊕⊝⊝ low5 |
Intratympanic therapy may increase the risk of vertigo/dizziness of unspecified timing as compared to systemic corticosteroid. |
| Vertigo/dizziness: at the time of injection | Comparison not applicablec | 3 studies reported a rate between 1.5% (1/67) and 21% (4/19) for those who received an intratympanic injectione | 301 (4 studies) | Not calculable | ⊕⊝⊝⊝ very low6 |
The evidence is very uncertain regarding the risk of vertigo/dizziness at the time of intratympanic injection of corticosteroid as primary treatment. |
| Ear pain: timing not reportedf | 4/141 (2.8%) | 74/148 (50%) | 289 (2 studies) | RR 15.68 (95% CI 6.22 to 39.49) | ⊕⊕⊕⊝ moderate7 | Intratympanic corticosteroid injection probably increases the risk of ear pain of unspecified timing as compared to systemic corticosteroid when used as primary treatment. |
| Ear pain: at the time of injectionf | Comparison not applicablec | 3 studies reported a rate between 4.8% (5/104) and 27.1% (35/129) | 393 (3 studies) | Not calculable | ⊕⊕⊝⊝ low8 | The evidence suggests that there may be a risk of ear pain at the time of intratympanic injection of corticosteroid as primary treatment. |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PTA: pure tone audiometry; RR: risk ratio | ||||||
|
aFourteen studies recruited participants suffering from sudden sensorineural hearing loss. The incidence of improvement for the systemic corticosteroid group in these 14 studies was 73.07%. We have used 731 per 1000 to express the assumed risk. bOnly the most widely reported adverse events are described here. For adverse events that could feasibly occur in either group, we have only included the studies that provided a rate for both groups. For adverse events that could only occur in one group, we have only included the studies that reported the rate in that group, and presented these as a range. A full description of adverse event data is available for reference in Table 2. cComparisons between patients receiving intratympanic therapy and those receiving only systemic therapy were regarded as invalid for the following adverse events: persistent tympanic membrane perforation, vertigo observed at the time of intratympanic injection and ear pain observed at the time of intratympanic injection. This is explained in Data extraction and management. dA single study reported a rate for both intratympanic and systemic corticosteroid (Rauch 2011). However, it is not specified whether all of the patients in the intratympanic corticosteroid group experiencing vertigo did so at the time of injection. We have therefore reported this outcome separately from vertigo/dizziness interpreted as having occurred specifically at the time of injection. eIn two studies, two groups received intratympanic injection: in Tsounis 2018, one group received intratympanic corticosteroid and the other received intratympanic and systemic corticosteroid; in Huang 2021, one group received intratympanic corticosteroid and the other received intravenous followed by intratympanic corticosteroid. fIn each study contributing data, the number of participants with ear pain/earache was presented separately from the numbers with ear pain at intratympanic injection. It was assumed, therefore that those participants with pain at injection were not included among those with ear pain/earache. GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to risk of bias: eight studies were at high risk of other bias, three studies were at risk of attrition bias and three studies were at risk of selection bias. Downgraded one level due to inconsistency: the size and direction of effect varied between the studies and the I2 value was 80%.
2Downgraded one level due to risk of bias: we judged 11 of 14 studies to be at unclear or high risk of selection bias and we judged 12 of 14 studies to be at high risk of other bias.
3Downgraded one level due to risk of bias: we judged six studies to be at high risk of other bias; two studies were at high risk of selection bias. Downgraded one level due to inconsistency: the I2 value was moderate (41%).
4Downgraded one level due to risk of bias: we judged one study to be at high risk of bias because of concern about random sequence generation and allocation concealment. Downgraded two levels due to imprecision: the number of events is smaller than the optimal information size (taken as 300 events) and an effect estimate could not be calculated.
5Downgraded one level due to imprecision: the number of events is smaller than the optimal information size (taken as 300 events). Downgraded one level because of indirectness: provision of data by only a single study from a single setting, which may not adequately represent all patients with ISSNHL.
6Downgraded two levels due to risk of bias: we judged two studies to be at high risk of bias because of incomplete outcome data; we judged one study to be at high risk of bias because of concern about random sequence generation and allocation concealment. Downgraded two levels due to imprecision: the number of events is smaller than the optimal information size (taken as 300 events) and an effect estimate could not be calculated.
7Downgraded one level due to imprecision: the number of events is smaller than the optimal information size (taken as 300 events).
8Downgraded two levels because of imprecision: the number of events is smaller than the optimal information size (taken as 300 events) and an effect estimate could not be calculated.
1. Adverse events: intratympanic compared to systemic corticosteroids as primary therapy.
| Adverse event reported | Study | How reported | Details of recovery | Rate in intervention group (%) | Rate in comparator group (%) | RR (95% CI) |
| Tympanic membrane (TM) perforation | Huang 2021* | There were no cases of [...] perforation of the tympanic membrane | NA | 0/52 (0) | 0/52 (0)* | NA |
| Kosyakov 2011 | No residual TM perforations | All patients demonstrated a complete healing of TM after the tympanostomy tube removal | 0/24 (0) | NA | NA | |
| Rauch 2011 | Persistent TM perforation | By the 6‐month follow‐up most adverse events had resolved | 5/129 (3.9) | NA | NA | |
| Tong 2021 | No residual tympanic membrane perforations were observed in any of the individuals at their final visit | NA | 0/30 (0) | NA | NA | |
| Vertigo/dizziness | Ermutlu 2017 | Four patients in the ITS group had transient vertigo during the procedure | NR | 4/19 (21) | NA | NA |
| Huang 2021* | [...] complained of brief dizziness after IT injection [...] |
No patients stopped the treatment | 7/52 (13.5) | 8/52 (15.4)* | NA | |
| Rauch 2011 | The intratympanic group experienced adverse effects typical of local injection, most often transient pain at the injection site and brief caloric vertigo. Note, it is unclear whether all reported instances of vertigo in the intervention group occurred at the time of injection. | By the 6‐month follow‐up most adverse events had resolved | 35/129 (27.1) | 13/121 (10.7) | 2.53 (1.41 to 4.54); favours systemic corticosteroid; P = 0.002 | |
| Swachia 2016 | Temporary adverse events in 22.7% of patients treated with oral prednisolone which included [...] and dizziness. In 35% of patients treated with intratympanic corticosteroid, adverse events occurred including [...] and dizziness. | NR | NR | NR | NA | |
| Tong 2021 | Six of 30 patients in the intratympanic injection group complained of a transient dizziness lasting about a minute during treatment | NR | 6/30 (20) | NA | NA | |
| Tsounis 2018 | One patient experienced transient dizziness as a result of caloric stimulation from the injected steroid solution (unclear which of 2 groups receiving intratympanic injection) | Symptoms resolved completely within 15 minutes and there was no need to discontinue the treatment. The injections that followed caused no further side effect. | NR | NR | NA | |
| Tinnitus | Swachia 2016 | In 35% of patients treated with intratympanic corticosteroid, adverse events occurred including [...] ringing sensation in the ear | NR | NR | NR | NA |
| Ear pain | Al‐Shehri 2015 | Pain due to injection | NR | 2/19 (10.5) | NA | NA |
| Earache | NR | 4/19 (21.1) | 0/20 (0) | 9.45 (0.54 to 164.49); favours systemic corticosteroid; P = 0.12 | ||
| Huang 2021 | [...] refused repeated IT injections due to unbearable pain | NR | 3/52 (5.8) | 2/52 (3.8) | NA | |
| Rauch 2011 | The intratympanic group experienced adverse effects typical of local injection, most often transient pain at the injection site [...] | By the 6‐month follow‐up most adverse events had resolved | 35/129 (27.1) | NA | NA | |
| Experienced ear pain at least once | By the 6‐month follow‐up most adverse events had resolved | 70/129 (54.3) | 4/121 (3.3) | 16.41 (6.18 to 43.59); favours systemic corticosteroid; P < 0.00001 | ||
| Swachia 2016 | In 35% of patients treated with intratympanic corticosteroid adverse events occurred including […] mild ear pain, severe ear pain (3 patients) | NR | 3/20 (15) severe ear pain | NR | NA | |
| Tong 2021 | Some patients had a tolerable pain reaction after the injection | NR | NR | NA | NA | |
| Other | Al‐Shehri 2015 | Mood change | NR | 2/19 (10.5) | 8/20 (40) | 0.26 (0.06 to 1.08); favours IT corticosteroid; P = 0.06 |
| Blood glucose problem | NR | 3/19 (15.8) | 6/20 (30) | 0.53 (0.15 to 1.81); favours IT corticosteroid; P = 0.31 | ||
| Sleep change | NR | 1/19 (5.3) | 6/20 (30) | 0.18 (0.02 to 1.32); favours IT corticosteroid; P = 0.09 | ||
| Increased appetite | NR | 1/19 (5.3) | 5/20 (25) | 0.21 (0.03 to 1.64); favours IT corticosteroid; P = 0.14 | ||
| Mouth dryness/thirst | NR | 0/19 (0) | 5/20 (25) | 0.10 (0.01 to 1.62); favours IT corticosteroid; P = 0.10 | ||
| Weight gain | NR | 0/19 (0) | 3/20 (15) | 0.15 (0.01 to 2.72); favours IT corticosteroid; P = 0.20 | ||
| Dispenza 2011 | No complications related to the treatment were noted in both the groups | NA | 0/25 (0) | 0/21 (0) | NA | |
| Ermutlu 2017 | No long‐term complications were observed in any of the patients | NA | 0/19 (0) | 0/16 (0) | NA | |
| Hong 2009 | No side effects were observed in either group | NA | 0/32 (0) | 0/31 (0) | NA | |
| Huang 2021* | Apparent bleeding at intratympanic injection site | NR | 0/52 (0) | 0/52 (0) | NA | |
| External otitis or myringitis | NR | 0/52 (0) | 0/52 (0) | NA | ||
| Otitis media | NR | 0/52 (0) | 0/52 (0) | NA | ||
| Fluctuation of basal blood pressure (> 10 mmHg) | NR | 2/52 (3.8) | 7/52 (13.5) | NA | ||
| Fluctuation of fasting blood glucose (> 2 mmol/L) | NR | 5/52 (9.6) | 12/52 (23.1) | NA | ||
| Emotional change | NR | 8/52 (15.4) | 15/52 (28.8) | NA | ||
| Appetite change | NR | 13/52 (25.0) | 25/52 (48.1) | NA | ||
| Dyssomnia | NR | 23/52 (44.2) | 38/52 (73.1) | NA | ||
| Water‐sodium retention | NR | 9/52 (17.3) | 24/52 (46.1) | NA | ||
| Acne on face and body | NR | 2/52 (3.8) | 6/52 (11.5) | NA | ||
| Irregular menstruation | NR | 5/21 (23.8) | 11/23 (47.8) | NA | ||
| Cushing's syndrome | NR | 0/52 (0) | 1/52 (1.9) | NA | ||
| Osteoporotic fracture | 0 | 0 | ||||
| Kosyakov 2011 | In one case an acute suppurative otitis media developed that was eliminated by local antibacterial therapy. This patient was excluded from the study. | NA | NA | NA | NA | |
| Nine patients in the ST (standard therapy) group and 12 patients in the intravenous corticosteroid group complained of sleep loss | Completely corrected after withdrawal | NR | 9/24 (37.5) in ST group and 12/25 (48) in intravenous corticosteroid group | NA | ||
| No systemic adverse effects related to intratympanic application of steroids were noticed | NA | 0/24 (0) | NA | NA | ||
| No serious side effects related to systemic administration of steroids were observed in the study | NA | NA | 0/49 (0) | NA | ||
| Qu 2015 | No complications were seen in patients (unclear which group), including those with hypertension or diabetes | NA | NR | NR | NA | |
| Rauch 2011 | Mood change | By the 6‐month follow‐up most adverse events had resolved | 12/129 (9.3) | 54/121 (44.6) | 0.21 (0.12 to 0.37); favours IT corticosteroid; P < 0.00001 | |
| Blood glucose problem | By the 6‐month follow‐up most adverse events had resolved | 21/129 (16.3) | 36/121 (29.8) | 0.55 (0.34 to 0.88); favours IT corticosteroid; P = 0.01 | ||
| Sleep change | By the 6‐month follow‐up most adverse events had resolved | 9/129 (7) | 44/121 (36.4) | 0.19 (0.1 to 0.38); favours IT corticosteroid; P < 0.00001 | ||
| Appetite change | By the 6‐month follow‐up most adverse events had resolved | 6/129 (4.7) | 28/121 (23.1) | 0.2 (0.09 to 0.47); favours IT corticosteroid; P = 0.0002 | ||
| Dry mouth/thirst | By the 6‐month follow‐up most adverse events had resolved | 5/129 (3.9) | 30/121 (24.8) | 0.16 (0.06 to 0.39); favours IT corticosteroid; P < 0.0001 | ||
| Weight change | By the 6‐month follow‐up most adverse events had resolved | 7/129 (5.4) | 22/121 (18.2) | 0.3 (0.13 to 0.67); favours IT corticosteroid; P = 0.004 | ||
| Ear infection | By the 6‐month follow‐up most adverse events had resolved | 7/129 (5.4) | 2/121 (1.7) | 3.28 (0.7 to 15.49); favours systemic corticosteroid; P = 0.13 | ||
| Any adverse event: "Adverse events were reported by 87.6% (106 of 121) of participants in the oral group and 89.9% (116 of 129) in the intratympanic group." Note: it is unclear whether 'adverse events' refers to those already reported (and listed for this study in this table). | By the 6‐month follow‐up most adverse events had resolved | 116/129 (89.9) | 106/121 (87.6) | 1.03 (0.94 to 1.12); favours systemic corticosteroid; P = 0.56 |
||
| Serious adverse events: "In the intratympanic treatment group, these included osteomyelitis of the toe, leukemia, myocardial infarction, bladder cancer, chest pain due to possible endocarditis, and exacerbation of pre‐existing chronic obstructive pulmonary disease. In the oral treatment group, the serious adverse events were myocardial infarction, cerebral hemorrhage, hyponatremia, hospitalization for possible transient ischemic attack, and syncope. The case of hyponatremia arose from worsening of pre‐existent mild renal insufficiency in a patient with type 2 diabetes that was deemed study‐related." | NR | 6/129 (4.7) | 5/121 (4.1) | 1.13 (0.35 to 3.59); favours systemic corticosteroid; P = 0.84 |
||
| Rupasinghe 2017 | No adverse effects were reported in either study group during the study period | NA | NA | NA | NA | |
| Swachia 2016 | Temporary adverse events: temporary adverse events in 22.7% of patients treated with oral prednisolone which included puffiness of face, ulcers in mouth, increased appetite, diarrhea [...] | NR | NR | 5/22 (22.7) | NA | |
| Adverse events: in 35% of patients treated with intratympanic methylprednisolone adverse events occurred including mild ear pain, severe ear pain (3 patients), ringing sensation in ear and dizziness | NR | 7/20 (35) | NR | NA | ||
| Tong 2021 | No otitis media […] observed in any of the individuals at their final visit | NA | 0/30 (0) | 0/30 (0) | NA | |
| Tsounis 2018 | No significant complications during the intratympanic injections or the follow‐up period | NA | 0/33 (0) | NA | NA |
*Patients in the comparator group for this trial also received intratympanic (IT) corticosteroid at a later time point in the trial, therefore complications of IT treatment are included here (Huang 2021). The intervention group received 24 days of IT corticosteroid and the comparator group received 12 days of systemic (intravenous corticosteroid) followed by 12 days of IT corticosteroid. Rate ratios are not presented as they are not applicable to the comparison of interest (IT compared to systemic as primary therapy).
CI: confidence interval; IT: intratympanic; NA: not applicable; NR: not reported; RR: risk ratio; TM: tympanic membrane
Summary of findings 2. Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as primary therapy.
| Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as primary therapy | ||||||
|
Patient or population: sudden sensorineural hearing loss Settings: initial therapy Intervention: combination of intratympanic and systemic steroid therapy Comparison: systemic steroid therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | No of participants (studies) | Relative effect (95% CI) | Certainty of the evidence (GRADE) | Comments | |
|
Systemic therapy (assumed risk) |
Combined therapy (corresponding risk) |
|||||
| Change in hearing threshold determined by PTA Range 0 dB to 140 dB Negative values represent lowering and positive values represent raising of the hearing threshold. A lower hearing threshold represents hearing improvement. |
The mean change in PTA ranged across control groups from ‐33.0 dB to ‐13.0 dB | The mean change in PTA in the intervention groups was on average ‐8.55 dB greater (‐4.61 greater to ‐12.48 greater) | 435 (6 studies) |
MD ‐8.55 dB (95% CI ‐12.48 to ‐4.61) |
⊕⊕⊝⊝ low1 |
The change in hearing threshold may be slightly increased in participants who receive combined therapy. However, it is unclear whether this increase would be noticeable to patients. |
| Proportion of patients whose hearing is improved | 579 per 1000a | 735 per 1000 (666 to 816) | 788 (10 studies) |
RR 1.27 (95% CI 1.15 to 1.41) |
⊕⊝⊝⊝ very low2 | The evidence is very uncertain as to whether combined therapy changes the proportion of participants whose hearing is improved. |
| Final hearing threshold determined by PTA A lower value represents better hearing |
The mean final PTA ranged across control groups from 39.1 dB to 59 dB | The mean final PTA in the intervention groups was on average 9.11 dB lower (1.67 lower to 16.56 lower) | 194 (3 studies) |
MD ‐9.11 dB (95% CI ‐16.56 to ‐1.67) |
⊕⊝⊝⊝
very low3 |
Combined therapy may result in slightly lower (more favourable) final hearing thresholds compared to systemic corticosteroids alone (as primary therapy) but the evidence is very uncertain, and it is not clear whether the change would be of importance to patients. |
| Adverse eventsb | Events in control group | Events in intervention group | No of Participants (studies) | Relative effect (95% CI) | Certainty of the evidence (GRADE) | Comments |
| Persistent tympanic membrane perforation | Comparison not applicablec | 5 studies reported a rate between 0% (0/85) and 5.5% (2/36) for those who received an intratympanic injection | 474 (5 studies) | Not calculable | ⊕⊝⊝⊝ very low4 |
The evidence is very uncertain regarding the risk of tympanic membrane perforation for those who received intratympanic steroids. |
| Vertigo/dizziness: timing not reported | No study reported on this outcome for both the intervention and comparator groups. | |||||
| Vertigo/dizziness: at the time of injection | Comparison not applicablec | 4 studies reported a rate between 0% (0/60) and 8.1% (3/37) for those who received an intratympanic injectiond | 341 (4 studies) | Not calculable | ⊕⊝⊝⊝ very low5 |
The evidence is very uncertain regarding the risk of vertigo/dizziness at the time of intratympanic injection for those who received intratympanic corticosteroid as primary treatment. |
| Ear pain: timing not reported | No study reported on this outcome for both the intervention and comparator groups. | |||||
| Ear pain: at the time of injection | Comparison not applicablec | One study reported a rate of 5/37 (13.5%) | 73 (1 study) | Not calculable | ⊕⊝⊝⊝ very low6 |
The evidence is very uncertain regarding the risk of ear pain at the time of intratympanic injection for those who received combined treatment as primary treatment. |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PTA: pure tone audiometry; RR: risk ratio | ||||||
|
aTen studies recruited participants suffering from sudden sensorineural hearing loss. The incidence of improvement for the 10 studies was 57.86%. We have used 579 per 1000 to express the assumed risk. bOnly the most widely reported adverse events are described here. For adverse events that could feasibly occur in either group, we have only included the studies that provided a rate for both groups. For adverse events that could only occur in one group, we have only included the studies that reported the rate in that group, and presented these as a range. A full description of adverse event data is available for reference in Table 4. cComparisons between patients receiving intratympanic therapy and those receiving only systemic therapy were regarded as invalid for the following adverse events: persistent tympanic membrane perforation, vertigo observed at the time of intratympanic injection and ear pain observed at the time of intratympanic injection. This is explained in Data extraction and management. dIn one study, two groups received intratympanic injection: one group received intratympanic corticosteroid and the other received intratympanic and systemic corticosteroid (Tsounis 2018). GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to risk of bias: we rated a study contributing moderate weight to the overall effect estimate as high risk of bias due to concern about random sequence generation and allocation concealment. Five studies were at high risk of other bias, and one study was at risk of attrition bias. Downgraded one level due to imprecision: the 95% CI overlaps the threshold for clinical relevance, taken to be 10 dB.
2Downgraded one level due to risk of bias: we judged 8 of 10 studies to be at high or unclear risk of selection bias and at high risk of other bias. Downgraded one level due to imprecision: the 95% CI overlaps the threshold for clinical relevance. Downgraded one level due to inconsistency: the I2 value was moderate (47%).
3Downgraded two levels due to risk of bias: we judged all three studies to be at high or unclear risk of selection bias and high risk of other bias. We also judged one of three studies to be at high risk of bias for incomplete outcome data and selective reporting. Downgraded one level due to imprecision: the 95% CI overlaps the threshold for clinical relevance and the sample size is smaller than the optimal information size (taken as 400 participants).
4Downgraded two levels due to risk of bias: we judged two studies to be at high risk of bias because of concern about random sequence generation, two studies because of selective reporting, one study because of concern about blinding and one study because of concern about allocation concealment.
5Downgraded two levels due to risk of bias: we judged one study to be at high risk of bias because of concern about random sequence generation and blinding, one study because of selective reporting and one study because of incomplete outcome data. Downgraded two levels due to imprecision: the number of events is smaller than the optimal information size (taken as 300 events) and an effect estimate could not be calculated.
6Downgraded two levels due to imprecision: the number of events is smaller than the optimal information size (taken as 300 events) and an effect estimate could not be calculated. Downgraded one level because of indirectness: provision of data by only a single study from a single setting, which may not adequately represent all patients with ISSNHL.
2. Adverse events: combined compared to systemic as primary therapy.
| Adverse event reported | Study | How reported | Details of recovery | Rate in intervention group (%) | Rate in comparator group (%) | RR (95% CI) |
| TM perforation | Ahn 2008 | No significant complications during or after IT dexamethasone, including TM perforation […] | NR | 0/60 (0) | NA | NA |
| Arastou 2013 | Two patients developed tympanic perforation (reported as 2.6% of whole study sample; unclear how many from each group) | Treated with cauterisation and paper patch (n = 1) and tympanoplasty (n = 1) | NR | NR | NA | |
| Arslan 2011 | None of the patients had an important complication, namely […] TM perforation (unclear which group) | NR | NR | NR | NA | |
| Choi 2011 | No significant complications during or after IT steroid injection including TM perforation | NR | 0/19 (0) | NA | NA | |
| Gundogan 2013 | No case of residual TM perforation […] was noted | No long‐term complications resulting from either oral steroid or IT steroid in any of the patients | 0/37 (0) | NA | NA | |
| Vertigo/dizziness | Ahn 2008 | No significant complications during or after IT dexamethasone, including […] vertigo […] | NR | 0/60 (0) | NA | NA |
| Arslan 2011 | None of the patients had an important complication, namely, […] vertigo […] (unclear which group) | NR | NR | NR | NA | |
| Choi 2011 | No significant complications during or after IT steroid injection, including […] vertigo […] | NR | 0/19 (0) | NA | NA | |
| Gundogan 2013 | Three patients complained of vertigo immediately after injection | Recovered after 2 hours of rest | 3/37 (8.1) | NA | NA | |
| Tsounis 2018 | One patient experienced transient dizziness as a result of caloric stimulation from the injected steroid solution (unclear which of two groups receiving intratympanic injection) | Symptoms resolved completely within 15 minutes and there was no need to discontinue the treatment. The injections that followed caused no further side effects. | NR | NR | NA | |
| Tinnitus | Ahn 2008 | No significant complications during or after IT dexamethasone, including […] and tinnitus | NR | 0/60 (0) | NA | NA |
| Arslan 2011 | None of the patients had an important complication, namely, […] tinnitus […] (unclear which group) | NR | NR | NR | NA | |
| Choi 2011 | No significant complications during or after IT steroid injection, including […] tinnitus […] | NR | 0/19 (0) | NA | NA | |
| Ear pain | Arslan 2011 | None of the patients had an important complication, namely, […] otalgia […] (unclear which group) | NR | NR | NR | NA |
| Gundogan 2013 | Otalgia occurred in 5 patients after IT corticosteroid injection | Relieved after 1 hour | 5/37 (13.5) | NA | NA | |
| Other | Ahn 2008 | No significant complications during or after IT dexamethasone, including […] otitis media […] | NR | 0/60 (0) | NA | NA |
| Arslan 2011 | None of the patients had an important complication, namely, […] nystagmus, otitis media […] (unclear which group) | NR | NR | NR | NA | |
| Battaglia 2008 | No long‐term complications resulted from either the prednisone taper or the IT corticosteroid in any of the patients enrolled in the study | NR | 0/16 (0) | 0/18 (0) | NA | |
| Choi 2011 | No significant complications during or after IT steroid injection, including […] otitis media […] | NR | 0/19 (0) | NA | NA | |
| Gundogan 2013 | No case of […] otitis media was noted | No long‐term complications resulted from either oral steroid or IT steroid in any of the patients | 0/37 | NA | NA | |
| No long‐ term complications resulted from either oral steroid or IT steroid in any of the patients | NA | 0/37 (0) | 0/36 (0) | NA | ||
| Tsounis 2018 | No significant complications during the intratympanic injections or the follow‐up period | NA | 0/33 (0) | NA | NA | |
| Koltsidopoulos 2013 | No significant complications occurred during IT injections or the follow‐up period. One case of otitis media was encountered (unclear which group) | NR | 0/46 (0) (significant complications) | NA | NA |
IT: intratympanic; NA: not applicable; NR: not reported; TM: tympanic membrane
Summary of findings 3. Intratympanic corticosteroids versus no treatment or versus placebo as secondary therapy.
| Intratympanic corticosteroids versus no treatment or versus placebo as secondary therapy | ||||||
|
Patient or population: sudden sensorineural hearing loss Settings: after treatment failure with systemic steroids Intervention: intratympanic steroid therapy Comparison: no treatment/placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | No of participants (studies) | Relative effect (95% CI) | Certainty of the evidence (GRADE) | Comments | |
|
No treatment/placebo (assumed risk) |
Intratympanic therapy (corresponding risk) |
|||||
| Change in hearing threshold determined by PTA Range 0 dB to 140 dB Negative values represent lowering and positive values represent raising of the hearing threshold. A lower hearing threshold represents hearing improvement. |
The mean change in PTA ranged across control groups from ‐13.21 dB to 0.8 dB | The mean change in PTA in the intervention groups was on average ‐9.07 dB greater (‐6.66 greater to ‐11.47 greater) | 280 (7 studies) |
MD ‐9.07 dB (95% CI ‐11.47 to ‐6.66) | ⊕⊕⊝⊝
low1 |
Intratympanic therapy may have a small effect on hearing threshold compared to no treatment or placebo (as secondary therapy), but it is not clear whether this change would be important to patients. |
| Proportion of patients whose hearing is improved | 70 per 1000a | 385 per 1000 (203 to 747) | 232 (6 studies) |
RR 5.55 (95% CI 2.89 to 10.68) |
⊕⊕⊝⊝ low2 | Intratympanic therapy may result in a much higher proportion of patients whose hearing is improved, compared to no treatment or placebo (as secondary therapy). |
| Final hearing threshold determined by PTA (a lower value represents better hearing) | The mean final PTA ranged across control groups from 59.9 to 90.5 dB HL | The mean final PTA in the intervention groups was on average ‐11.09 dB lower (‐4.72 lower to ‐17.46 lower) | 203 (5 studies) |
MD ‐11.09 dB (95% CI ‐17.46 to ‐4.72) |
⊕⊕⊝⊝ low3 | Intratympanic therapy may result in lower (more favourable) final hearing thresholds compared to no treatment or placebo (as secondary therapy). |
| Adverse eventsb | Events in control group | Events in intervention group | No of Participants (studies) | Relative effect (95% CI) | Certainty of the evidence (GRADE) | Comments |
| Persistent tympanic membrane perforation | Comparison not applicablec | 5 studies reported a rate between 0% (0/19) and 4.2% (1/24) for those who received an intratympanic injectiond | 185 (5 studies) | Not calculable | ⊕⊝⊝⊝ very low4 |
The evidence is very uncertain regarding the risk of tympanic membrane perforation for those who received intratympanic injection (either corticosteroid or placebo) as secondary treatment. |
| Vertigo/dizziness: timing not reported | No study reported on this outcome for both the intervention and comparator groups. | |||||
| Vertigo/dizziness at the time of intratympanic injection | Comparison not applicablec | 3 studies reported a rate between 6.7% (1/15) and 33% (number not reported) for those who received an intratympanic injection.d | 128 (3 studies) | Not calculable | ⊕⊝⊝⊝ very low5 |
The evidence is very uncertain regarding the risk of vertigo/dizziness at the time of intratympanic injection (either corticosteroid or placebo) as secondary treatment. |
| Ear pain: timing not reported | No study reported on this outcome for both the intervention and comparator groups. | |||||
| Ear pain at the time of intratympanic injection | Comparison not applicablec | One study reported no participants with ear pain at the time of intratympanic injection (0/24) | 44 (one study) | Not calculable | ⊕⊝⊝⊝ very low6 |
The evidence is very uncertain regarding the risk of ear pain at the time of intratympanic corticosteroid injection as secondary treatment. |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PTA: pure tone audiometry; RR: risk ratio | ||||||
|
aSix studies recruited participants suffering from sudden sensorineural hearing loss after treatment failure with systemic steroids. The incidence of improvement for the control group in these six studies was 6.96%. We have used 70 per 1000 to express the assumed risk. bOnly the most widely reported adverse events are described here. For adverse events that could feasibly occur in either group, we have only included the studies that provided a rate for both groups. For adverse events that could only occur in one group, we have only included the studies that reported the rate in that group, and presented these as a range. A full description of adverse event data is available for reference in Table 6. cComparisons between patients receiving intratympanic therapy and those receiving only systemic therapy were regarded as invalid for the following adverse events: persistent tympanic membrane perforation, vertigo observed at the time of intratympanic injection and ear pain observed at the time of intratympanic injection. This is explained in Data extraction and management. dThis includes participants who received placebo intratympanic injection. GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to risk of bias: we rated one study contributing moderate weight to the overall effect estimate as having high risk of bias due to incomplete outcome data. All studies were at high risk of other bias. Downgraded one level due to imprecision: the 95% CI for the effect overlaps the threshold for clinical relevance and the sample size is smaller than the optimal information size (taken as 400 participants). One study included treatment in the comparator arm with vitamin B, vasodilators and benzodiazepines (Ho 2004). However, as the weight of this study in the meta‐analysis was low and exclusion of the study made little difference to the effect estimate we did not downgrade for indirectness.
2Downgraded one level due to risk of bias: we rated two studies as being at high risk of bias due to selective reporting and one study was at high risk of bias for incomplete outcome data. All studies were at high risk of other bias. Downgraded one level due to imprecision: the total number of events is smaller than the optimal information size (taken as 300 events).
3Downgraded one level due to risk of bias: we rated one study contributing moderate weight to the overall effect estimate as high risk of bias due to incomplete outcome data, and one other study as high risk of bias because of selective reporting. All studies were at high risk of other bias. Downgraded one level due to imprecision: the 95% CI for the effect overlaps the threshold for clinical relevance and the sample size is smaller than the optimal information size (taken as 400 participants).
4Downgraded one level due to risk of bias: we rated one study as high risk of bias because of selective reporting; we rated one study as high risk of bias because of incomplete outcome data; we rated three studies as uncertain for random sequence generation, allocation concealment and blinding. Downgraded two levels because of imprecision: the number of events is smaller than the optimal information size (taken as 300 events) and an effect estimate could not be calculated.
5Downgraded one level due to risk of bias: we rated one study as high risk of bias because of selective reporting; we rated one study as high risk of bias because of incomplete outcome data; we rated two studies as uncertain for random sequence generation, allocation concealment and blinding. Downgraded two levels because of imprecision: the number of events is smaller than the optimal information size (taken as 300 events) and an effect estimate could not be calculated.
6Downgraded two levels because of imprecision: the number of events is smaller than the optimal information size (taken as 300 events) and an effect estimate could not be calculated. Downgraded one level due to indirectness: single study from a single setting, which may not adequately represent all patients with ISSNHL.
3. Adverse events: intratympanic compared to no treatment/placebo as secondary therapy.
| Adverse event reported | Study | How reported | Details of recovery | Rate in intervention group (%) | Rate in comparator group (%) | RR (95% CI) |
| TM perforation | Ho 2004 | No residual TM perforation | NA | 0/15 (0) | NA | NA |
| Li 2011 | Persistent TM perforation | No hearing loss in the affected ear. The perforation was treated successfully with a paper patch. | 1/24 (4.2) | NA | NA | |
| Plontke 2009 | One patient (unclear which group) had a major catheter dislocation with perforation of ear drum. Note: both groups received IT injection, either corticosteroid or normal saline. | Small ear drum perforation was closed with a myringoplasty | NR | NR | NA | |
| Wu 2011 | Transient TM perforation | Healed spontaneously by follow‐up 1 month later | 1/27 (3.7) | NR | NA | |
| Xenellis 2006 | No TM perforation was noticed at last visit | NA | 0/19 (0) | NA | NA | |
| Vertigo/dizziness | Ho 2004 | Complained of vertigo immediately after injection | Recovered after 2 hours of rest | 1/15 (6.7) | NA | NA |
| Li 2011 | Three patients complained of vertigo [...] during the injections | Resolved within minutes | 3/24 (12.5) | NA | NA | |
| No disequilibrium | NA | 0/24 (0) | NR | NA | ||
| No dizziness for more than 24 hours | NA | 0/24 (0) | NR | NA | ||
| Plontke 2009 | One patient (unclear which group) with increase in vertigo | Resolved | NR | NR | NA | |
| Wu 2011 | Temporary dizziness experienced by one‐third of subjects (unclear how many each group). Note: both groups received IT injection, either corticosteroid or normal saline. | Relieved by resting for a short time. Three participants quit the trial because of uncomfortable dizziness (unclear how many each group). | NR | NR | NA | |
| Tinnitus | Li 2011 | Three patients complained of vertigo or an increase in tinnitus during the injections | Resolved within minutes | 3/24 (12.5) | NA | NA |
| Hearing loss | Li 2011 | The injection did not induce an increase in […] hearing loss […] for greater than 24 h | NA | 0/24 (0) | NR | NA |
| Wu 2011 | No participant experienced a decrease in hearing of 10 dB or more | NA | 0/27 | 0/28 | NA | |
| Ear pain | Li 2011 | The injection did not induce an increase in ear pain […] | NA | 0/24 (0) | NA | NA |
| Plontke 2009 | Two patients (unclear how many each group) with ear pain. Note: both groups received IT injection, either corticosteroid or normal saline. | Resolved | NR | NR | NA | |
| Xenellis 2006 | A mild ear pain occurring the first hour post‐injection | Easily controlled with common analgesics | NR | NA | NA | |
| Other | Ho 2004 | One of 15 patients had acne | NR | 1/15 (6.7) | NR | NA |
| Li 2011 | No serious complications such as chronic otitis media, disequilibrium or dysgeusia developed | NA | 0/24 (0) | NR | NA | |
| Plontke 2009 | One patient (unclear which group) with each of: ear canal skin defect, steroid acne, nausea after antibiotic intake, gastroenteritis, hypokalaemia, pump battery failure and viral conjunctivitis. Three patients with headache (unclear how many in each group; one considered as 'possibly', 'probably' or 'very likely' related to the study) and 3 (unclear how many each group) with increased liver function tests (probably due to antibiotics). | Resolved | NR | NR | NA | |
| Wu 2011 | Severe nausea or vomiting was not experienced by any of the participants after the injection therapy | NA | 0/27 | 0/28 | NA | |
| Xenellis 2006 | No infection was noticed in any of the patients at their last visit | NA | 0/19 (0) | 0/18 (0) | NA |
TM: tympanic membrane; NA: not applicable; NR: not reported; IT: intratympanic
Summary of findings 4. Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as secondary therapy.
| Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as secondary therapy | ||||||
|
Patient or population: sudden sensorineural hearing loss Settings: after treatment failure with systemic steroids Intervention: combination of intratympanic and systemic steroid therapy Comparison: systemic steroid therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | No of participants (studies) | Relative effect (95% CI) | Certainty of the evidence (GRADE) | Comments | |
|
Systemic therapy (assumed risk) |
Combined therapy (corresponding risk) |
|||||
| Change in hearing threshold determined by PTA | No studies reported this outcome. | |||||
| Proportion of patients whose hearing is improved | 205 per 1000a | 459 per 1000 (226 to 933) | 76 (1 study) |
RR 2.24 (95% CI 1.10 to 4.55) |
⊕⊝⊝⊝ very low1 | Combined therapy may increase the proportion of patients whose hearing is improved compared to systemic corticosteroids alone (as secondary therapy), but the evidence is very uncertain. |
| Final hearing threshold determined by PTA | No studies reported this outcome. | |||||
| Adverse eventsb | Events in control group | Events in intervention group | No of participants (studies) | Relative effect (95% CI) | Certainty of the evidence (GRADE) | Comments |
| Persistent tympanic membrane perforation | Comparison not appropriatec | One study reported a rate of 8.1% (3/37) | 76 (1 study) | Not calculable | ⊕⊝⊝⊝ very low2 |
The risk of tympanic membrane perforation among those who receive intratympanic corticosteroid combined with systemic corticosteroid as primary treatment is very uncertain. |
| Vertigo/dizziness: timing not reported |
No studies reported this outcome. | |||||
| Vertigo/dizziness: at the time of injection |
Comparison not appropriatec | No study reported a rate in the intervention group. | ||||
| Ear pain: timing not reported |
No studies reported this outcome. | |||||
| Ear pain: at the time of injection |
Comparison not appropriatec | No study reported a rate in the intervention group. | ||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PTA: pure tone audiometry; RR: risk ratio | ||||||
|
aOne study recruited participants suffering from sudden sensorineural hearing loss after treatment failure with systemic steroids. The incidence of improvements was 20.51%. We have used 205 per 1000 to express the assumed risk. bOnly the most widely reported adverse events are described here. For adverse events that could feasibly occur in either group, we have only included the studies that provided a rate for both groups. For adverse events that could only occur in one group, we have only included the studies that reported the rate in that group, and presented these as a range. A full description of adverse event data is available for reference in Table 8. cComparisons between patients receiving intratympanic therapy and those receiving only systemic therapy were regarded as invalid for the following adverse events: persistent tympanic membrane perforation, vertigo observed at the time of intratympanic injection and ear pain observed at the time of intratympanic injection. This is explained in Data extraction and management. GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded two levels due to risk of bias: we judged the study to be at high risk of selection bias, performance bias, incomplete outcome data, selective reporting and other bias. Downgraded two levels due to imprecision: the 95% CI overlaps the threshold for clinical relevance and the total number of events is smaller than the optimal information size (taken as 300 events).
2Downgraded two levels due to risk of bias: we judged the study to be at high risk of bias because of selection bias, concern about blinding, incomplete outcome data and selective reporting. Downgraded two levels due to imprecision: the number of events is smaller than the optimal information size (taken as 300 events) and an effect estimate could not be calculated. Downgraded one level because of indirectness: provision of data by only a single study from a single setting, which may not adequately represent all patients with ISSNHL.
4. Adverse events: combined compared to systemic as secondary treatment.
| Adverse event reported | Study | How reported | Details of recovery | Rate in intervention group (%) | Rate in comparator group (%) | RR (95% CI) |
| TM perforation | Zhou 2011 | Three patients had small eardrum perforations | Successful closure by simple treatment | 3/37 (8.1) | NA | NA |
| Vertigo | Zhou 2011 | Second frequent complaint: transient vertigo after the drug had been injected into the ear | Not a severe problem if the drug was heated in 37°C water before injection and the vertigo disappeared after a few minutes or under 30 minutes | NR | NA | NA |
| Ear pain | Zhou 2011 | Most frequent complaint | Easily controlled by the oral administration of paracetamol 30 minutes before the local infusion of the methylprednisolone | NR | NA | NA |
| Hearing loss* | Zhou 2011 | No loss in hearing related to the treatment (in either group) | NA | 0/37 (0) | 0/39 (0) | NA |
| Other | Zhou 2011 | One patient had tongue paresthesia (unclear which group) | Resolved after 2 weeks | NR | NR | NA |
| No infections were observed (unclear which group) | NA | NR | NR | NA | ||
| Long‐term complications did not occur in any patients who received the transtympanic injections | NA | 0/37 (0) | NR | NA |
*Hearing loss defined as ≥ 15 dB worsening in pure tone audiometry or ≥ 15% worsening of speech discrimination score.
NA: not applicable; NR: not reported; TM: tympanic membrane
Background
Description of the condition
Idiopathic sudden sensorineural hearing loss (ISSNHL) is a sudden decrease in sensorineural hearing sensitivity of unknown aetiology. It is usually unilateral and the degree of severity can vary from mild hearing loss to total deafness. It may also be accompanied by vertigo and tinnitus.
There is no international consensus on the definition of ISSNHL in terms of the degree of hearing threshold change or the number of specific frequencies that are affected on pure tone audiological testing. A definition that is commonly used is "loss of at least 30 dB in three connected frequencies within 72 hours" (Chandrasekhar 2019; NIDCD 2018). However, this definition is not universally accepted. It does not specify the frequencies and the frequency range, the rational for choosing this threshold is not known and it is often not used as an inclusion criterion in clinical trials on ISSNHL. Although for mild and moderate hearing losses, statistical floor effects complicate the evaluation of recovery (Chen 2003), it appears justified to expand the definition to cases with less than 30 dB of hearing loss (Chandrasekhar 2019; Plontke 2007). There is also a lack of consensus on the most appropriate outcome criteria for clinical studies (Plontke 2007).
The incidence of sudden sensorineural hearing loss has been estimated to be 5 to 20 per 100,000 per year in industrial countries (Byl 1977; Hughes 1996; Stokroos 1996). However, according to studies in Germany, the incidence may be much higher: Olzowy 2005 estimated the incidence at 160 per 100,000 per year and Klemm 2009 estimated it at 400 per 100,000 per year. This discrepancy may be due to the absence of international consensus on the audiological definition and outcome criteria. The mean age of patients included in randomised controlled trials (RCTs) is between 45 and 55. Men and women are equally affected. ISSNHL in childhood is rare (Desloovere 1988; Klemm 2007; Mösges 2009; Plontke 2007; Probst 1992; Tucci 2002; Tran Ba Huy 2005). Idiopathic sudden sensorineural hearing loss, particularly when accompanied by tinnitus and dizziness, results in a significant reduction in quality of life (Carlsson 2011; Stachler 2012).
Various theories to explain ISSNHL have been proposed, for example viral infection, vascular occlusion, breakdown of labyrinthine membranes or barriers, immune‐mediated mechanisms (Vambutas 2021) and abnormal cellular stress responses within the cochlea. However, none of these hypotheses has been proven convincingly in humans (Merchant 2005; Merchant 2008).
Treatment modalities for ISSNHL are mostly based on the above etiopathogenetic hypotheses and include (gluco)corticosteroids, rheological drugs (e.g. dextran, hydroxyethyl starch, pentoxifylline and naftidrofuryl), vasodilators, anaesthetics, osmotically active substances, antioxidants, thrombocyte aggregation inhibitors, fibrinogen reduction through drugs or apheresis or rheopheresis (Suckfüll 2002), hyperbaric oxygen therapy, antiviral therapy, N‐methyl‐D‐aspartate (NMDA) receptor antagonists, immunosuppressants, anti‐apoptotic substances (Suckfuell 2014), and other substances (see reviews in: Conlin 2007a; Labus 2010; Lawrence 2015; Plontke 2005). Cochrane Reviews have assessed treatment of ISSNHL with systemic corticosteroids (Wei 2006; Wei 2013), hyperbaric oxygen (Bennett 2007; Bennett 2012), and vasodilators (Agarwal 2009), without demonstrating clear efficacy.
Systemic corticosteroids are widely used for ISSNHL worldwide (Plontke 2005). A Cochrane Review on systemic corticosteroids for ISSNHL found that there was uncertainty about the value of corticosteroids in the treatment of ISSNHL, "since the evidence from randomised controlled trials is contradictory in outcome, in part because the studies are based upon too small a number of patients" (Wei 2006). These findings were supported by another meta‐analysis (Conlin 2007b). The updated version of the Cochrane Review also included a randomised, placebo‐controlled, multicentre trial published in 2012 comparing the effect of prednisolone and placebo (Nosrati‐Zarenoe 2012); again the review found that there was uncertainty about the value of systemic corticosteroids in the treatment ISSNHL (Wei 2013).
In general, possible side effects of systemic corticosteroid medication include metabolic complications, such as glucose intolerance and diabetes mellitus, hypertension, increased intraocular pressure and glaucoma, psychotropic effects, hypothalamic‐pituitary‐adrenal‐axis suppression, gastrointestinal bleeding, bone loss, avascular necrosis of the femoral or humeral head and potential infections. A study investigating the risk of corticosteroid‐induced hyperglycaemia concluded that prevalence during systemic therapy is high and rises as the dose increases (Rohrmeier 2012). Although the rate of occurrence of side effects with systemic corticosteroid therapy appears low (Garcia‐Berrocal 2008), systematic data recording and publication of the proposed side effects are still insufficient, and adverse effects from a short course of high‐dose systemic corticosteroids have not been documented with good evidence. It is only possible, therefore, to speculate as to whether these known side effects occur during (longer) systemic corticosteroid treatment of ISSNHL and, if so, to what degree.
The terms 'steroids', 'corticosteroids', 'glucocorticoids' are unfortunately used imprecisely and interchangeably in the literature on ISSNHL. The term 'corticosteroid' is used throughout this review, since this term is more often used and generally accepted in the literature on ISSNHL (Chandrasekhar 2019; Rauch 2011).
Description of the intervention
The rationale for local intratympanic application of drugs for the treatment of inner ear diseases is based on the expected advantages over systemic treatment. These are 1) the bypassing of the blood‐labyrinthine barrier, resulting in 2) higher concentrations in the inner ear fluids despite the lower total amount of drug given, and 3) avoiding the major unwanted effects of systemically administered medications due to lower systemic drug levels.
Pharmacokinetic studies in animals and humans have shown that high doses of systemic corticosteroids are needed to achieve detectable drug levels in the inner ear perilymph and that substances applied to the round window membrane lead to significantly higher drug levels in the inner ear fluids compared to systemic application (Bachmann 2001; Bird 2007; Bird 2011; Chandrasekhar 2000; Niedermeyer 2003; Parnes 1999). Thus, applying drugs locally may be more effective in treating sudden sensorineural hearing loss and may avoid systemic complications and side effects. The introduction of this drug delivery approach has triggered a large number of pre‐clinical studies focused on the pharmacokinetics of local drug delivery to the inner ear and the development of drug delivery systems (reviewed, for example, in: Hoskison 2013; Nakagawa 2011; Pararas 2012; Salt 2009; Salt 2018).
Intratympanic injection of corticosteroids for ISSNHL in humans was pioneered by Silverstein (Silverstein 1996) and Parnes (Parnes 1999). Since then, a rapidly growing number of reports have been published on treatment results of intratympanic application of corticosteroids for inner ear disorders (Lavigne 2016; Liebau 2017; Liebau 2018). Intratympanic injection of corticosteroids is used not only as a single treatment approach but also in combination with systemic administration of corticosteroids. In a Cochrane meta‐analysis, Phillips 2011 assessed the efficacy of intratympanic corticosteroids for Ménière's disease. The majority of clinical reports, however, described the use of intratympanic corticosteroids for sudden hearing loss and more studies, including randomised controlled trials, are ongoing. So far, mainly dexamethasone or methylprednisolone preparations have been used as a primary or a second‐line ('rescue', 'salvage', 'reserve') intratympanic therapy for ISSNHL. Although these studies have shown intratympanic treatment with corticosteroids to be relatively safe, efficacy is difficult to assess since many studies did not compared their findings with a control group, and an even smaller number were randomised trials (reviewed in: Chandrasekhar 2019; Crane 2015; Gao 2016; Garavello 2012; Haynes 2007; Lavigne 2016; Lawrence 2015; Li 2015; Marx 2018 Ng 2015; Seggas 2011; Spear 2011; Stachler 2012; Vlastarakos 2012; Zhao 2016).
Several methods for intratympanic application of corticosteroids haven been developed in recent years. Most are single or repeated intratympanic injections with or without visualisation of the round window membrane. In some studies additional substances like hyaluronic acid are used for volume stabilisation to increase the persistence of the drug in the middle ear. Another strategy is continuous or discontinuous drug application via partly or fully implantable pump systems, allowing adjustment of drug concentrations over time. Potential adverse events in the intratympanic application of corticosteroids are in principle the same as in systemic drug administration, but to a lesser extent. Some of the intratympanically applied drug may be lost from the middle ear by drainage through the Eustachian tube and then swallowed. However, the dose is much lower than with systemic application. Persistent perforation of the tympanic membrane can develop after injection if there is an impaired healing process. Also, temporary pain may be observed, or temporary vertigo or dizziness due to caloric stimulation.
How the intervention might work
Corticosteroids were originally implemented in the treatment of ISSNHL because of their anti‐inflammatory effect. It is assumed that the main cause of sudden deafness is a harmful effect of the immune system on the inner ear in response to viral infection (Wilson 1980). However, corticosteroids have further effects, mainly mediated by activation of the glucocorticoid receptor, which could play a role in the treatment of sudden hearing loss. One important effect is an increase in anti‐apoptotic transcription factors in cells and the blocking of apoptosis signalling pathways. This could protect the sensory hair cells and other neural and non‐neural structures in the inner ear (Eshraghi 2006; Hoang 2009; Trune 2012). However, probably the most important effect of corticosteroids is their property of reducing the impact of oxidative stress in cells (Trune 2012). Recent studies point out that oxidative stress plays an important role in the aetiology of sudden hearing loss (Gul 2016; Quaranta 2016). Glucocorticoids also bind to the mineralocorticoid receptor (Grossmann 2004). Additional effects of corticosteroids are mediated by activation of the mineralocorticoid receptor, which has an impact on cochlear ion transport (Trune 2006). This may help to restore a disturbed homeostasis in the inner ear and ensure hair cell function driven by the endocochlear potential (MacArthur 2015). For some of these effects, especially the anti‐apoptotic and anti‐oxidative effects, a high drug concentration in the inner ear might be necessary (Haake 2009). Since there is no accumulation of corticosteroids in the inner ear, and as corticoid entrance to the inner ear structures is limited by the blood‐labyrinth barrier, drug concentration in the inner ear with systemic application will not exceed the systemic plasma concentration. Local application of corticosteroids to the inner ear by intratympanic injection achieves a short‐duration, high concentration of the drug in the middle ear cavity, from where the drug can diffuse through the boundaries to the inner ear, i.e. the round window and the oval window (King 2011; Salt 2009). Thus, higher corticosteroid concentrations can be achieved in the inner ear, which might be necessary for successful treatment of sudden hearing loss (Bird 2007; Bird 2011).
Hearing recovery in patients with ISSNHL mostly occurs early, within a few days of onset, but can also occur after several weeks (Liebau 2017; Liebau 2018). It has been observed that the time course of hearing recovery can extend to six months (Kosyakov 2011). However, most of the hearing improvement will take place during the first weeks after onset. To estimate the treatment effect of an intervention for ISSNHL, it is desirable that the final outcome assessment is conducted after several weeks of follow‐up. Short evaluation periods may risk underestimation of treatment effects (Wycherly 2011). After the recovery period, the hearing thresholds reached can be assumed to be stable in most patients. A large randomised controlled trial (published protocol) assessed the primary outcome 30 days after onset with an initial assessment after 10 days and a follow‐up at six months (Plontke 2016).
Why it is important to do this review
There is still uncertainty as to 1) whether intratympanic corticosteroids are better than placebo or no treatment, 2) whether intratympanic administration of corticosteroids alone or in combination with systemic application of steroids will lead to better results than systemic drug administration alone, 3) if so, which treatment protocol will lead to the best outcome and 4) what risks of adverse events are associated with this approach in inner ear therapy. This Cochrane Review was therefore warranted to assess the benefits and harms of intratympanic corticosteroids treatment for ISSNHL.
Objectives
To assess the effects of intratympanic corticosteroids in people with idiopathic sudden sensorineural hearing loss (ISSNHL).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐randomised controlled trials according to the Cochrane definition (Handbook 2021). Cross‐over trials were not included. Cross‐over trials are not feasible for the evaluation of interventions in the treatment of ISSNHL as there is no possibility to return to the baseline situation after the first intervention.
Types of participants
We included adults and children, female and male, of any ethnic origin, with unilateral ISSNHL (i.e. sudden sensorineural hearing loss of unknown aetiology) with or without vertigo, and with or without tinnitus.
Studies in patients with non‐idiopathic conditions or diagnoses were excluded (e.g. acoustic trauma, Ménière's disease, fluctuating hearing loss, endolymphatic hydrops, suspected retro‐cochlear lesion, hearing loss due to ear surgery, perilymph fistula or barotrauma, middle ear inflammation or effusion, or conductive hearing loss).
Types of interventions
Corticosteroids (also referred to as steroids), which were applied by intratympanic application for the treatment of ISSNHL as one of two treatment strategies:
as primary (first‐line) treatment; or
as secondary (rescue/salvage/reserve/second‐line) treatment after failure of primary therapy.
Corticosteroids were administered using one of the following drug delivery systems:
single or repeated intratympanic injection with or without volume stabilisation and with or without visualisation of the round window membrane; or
continuous or discontinuous drug application via partly or fully implantable pump systems.
The different methods of intratympanic drug delivery were considered together as intratympanic application.
We included studies of the following comparisons:
intratympanic corticosteroids versus no treatment or versus placebo;
intratympanic corticosteroids versus systemic corticosteroids;
intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone;
intratympanic plus systemic corticosteroids (combined therapy) versus no treatment or versus placebo.
Studies were included regardless of the precise details of the treatment protocol (e.g. type of corticosteroid used, injection procedure, dose, frequency of application and duration of treatment).
Types of outcome measures
We did not use the outcomes selected for the review as a basis for including or excluding studies. We conducted analyses on outcome data collected more than one week (eight days or more) after the start of treatment.
The primary outcome was the change in mean hearing threshold determined by pure tone audiometry (pure tone average) between treatment arms and measured in decibels (dB). A lowering of the mean hearing threshold represents an improvement in hearing. To indicate the direction of change, we denoted a lowering of mean threshold as a negative value, and an elevation of the mean threshold as a positive value. There was no restriction on frequencies or number of frequencies used for generation the pure tone average.
Secondary outcome measures included final hearing threshold (pure tone average at the study endpoint), frequency‐specific changes in mean hearing threshold, the proportion of patients whose hearing improved (based on pure tone average and/or speech audiometry and without restriction on definition) and changes in hearing threshold based on speech audiometry (without restriction on type or language of speech test).
Also among the secondary outcomes were minor and serious adverse events.
Primary outcomes
Change in hearing threshold with pure tone audiometry (pure tone average (PTA)).
Secondary outcomes
Proportion of patients whose hearing is improved (criteria for improvement were defined by the included studies).
Final hearing threshold with pure tone audiometry.
Change in hearing threshold with speech audiometry.
Frequency‐specific changes in hearing threshold with pure tone audiometry.
Mean level of improvement in those whose hearing is improved.
For patients with profound pre‐treatment hearing loss: percentage of patients reaching serviceable hearing (defined as maximum percentage of correctly understood monosyllables equal or greater than 50%).
Effect on tinnitus and vertigo.
Minor and serious adverse events.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 23 September 2021.
Electronic searches
The Information Specialist searched:
the Cochrane ENT Trials Register (searched via the Cochrane Register of Studies 23 September 2021);
the Cochrane Central Register of Controlled Trials (searched via the Cochrane Register of Studies) (CENTRAL 2021, Issue 9);
PubMed (1946 to 23 September 2021);
Ovid Embase (1974 to 23 September 2021);
LILACS, lilacs.bvsalud.org (searched 23 September 2021);
Web of Knowledge, Web of Science (1945 to 23 September 2021);
CNKI, www.cnki.com.cn (searched via Google Scholar 23 September 2021);
ClinicalTrials.gov (searched via the Cochrane Register of Studies and clinicaltrials.gov 23 September 2021);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), www.who.int/ictrp (searched 23 September 2021).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched PubMed, the Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Data collection and analysis
Data collection and analysis for this review were specified in a pre‐published protocol (Plontke 2009). Changes that have been made since the protocol are specified in the section Differences between protocol and review.
Selection of studies
After scanning all search results and independent screening of titles and abstracts, we retrieved the full texts of reports that loosely met the inclusion criteria and where exclusion of studies could not be clearly inferred from the abstract. At least two authors reviewed these and applied the inclusion criteria independently. These were 1) intratympanic corticosteroid treatment of ISSNHL, 2) clinical study, 3) stated randomisation process and 4) studying at least one comparison included in the review.
Final decisions on inclusion were based on full‐text analysis of preselected studies for the following criteria: 1) a reported randomisation process in the main text of the study report, 2) studying comparisons included in the review, 3) the diagnosis of included patients was ISSNHL, 4) the proportion of included patients with bilateral ISSNHL was below 5%, and 5) the time point of final outcome assessment was at least one week (eight days or more) after the start of treatment. In order to include a high number of studies, we also included those performing outcome assessments with short follow‐up (less than four weeks). However, we considered a short follow‐up duration of two weeks or less to represent a high risk of bias in these studies.
We openly discussed any differences of opinion about which studies to include in the review. If consensus could not be reached, we planned to refer these studies to the Cochrane ENT Co‐ordinating Editor. However, this was not necessary in any case. Publications in languages that could not be read by the authors were fully translated by a professional translator. Further, if such studies were included in the meta‐analyses, two native speakers independently performed extraction of key data, co‐ordinated by Cochrane ENT.
Data extraction and management
Study characteristics and data related to participants from each study were always independently extracted by at least three authors. Any discrepancies among the extracted data were discussed and resolved by consensus. Only treatment arms that met the comparisons defined in the review were included. If a study had more than one treatment arm matching the same type of intervention, we selected the one most widely used among included studies. In most studies, we extracted outcome data from the defined primary endpoint. When no time point was defined as the primary endpoint, we selected the latest time point for inclusion. Exceptionally, if the number of participants lost to follow‐up was very high at the final time point and the necessary outcome parameters and numbers of participants were reported for an earlier time point, we chose this earlier time point. This is documented in the Characteristics of included studies table.
We documented the following details for each study:
Methods (study design, country, year, setting, allocation, blinding).
Participants (inclusion and exclusion criteria, number of included participants, baseline parameters).
Interventions (treatment arms, time point of start of intervention, whether primary therapy or secondary/rescue therapy, dosage and type of steroid, drug delivery strategy, injection regime, duration of intervention, time to follow‐up, concomitant treatments).
Outcomes (defined primary and secondary outcomes in the review (see above), definition of PTA and successful hearing improvement, number of completed and analysed participants, type of analysis).
Funding sources and declarations of interest.
We always extracted outcome data based on intention‐to‐treat (ITT) analysis when they were reported, in preference to data based on per‐protocol (PP) analysis. We extracted the following summary statistics for each study and each outcome:
For continuous data: the mean values, standard deviations and number of patients for each treatment group.
For binary data: the number of participants experiencing an event and the number of patients assessed at the time point.
We took an exploratory approach to assessing adverse events and extracted data on all adverse events reported by the trialists.
Assessment of risk of bias in included studies
Assessment of the risk of bias of the included studies was undertaken independently by four authors with the following domains taken into consideration, as guided by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective reporting; and
other sources of bias.
We used the Cochrane risk of bias tool in RevMan 5 (RevMan 2020), which involves scrutiny of each domain as reported in the trial and judgement about the adequacy of each entry. Discrepancies between raters' judgements were discussed and resolved by consensus. We judged the risk of bias to be 'high', 'low' or 'unclear' and documented this together with an explanation in the risk of bias tables in Characteristics of included studies. In non‐placebo‐controlled studies, we generally considered the risk of bias derived from a lack of blinding to be 'low' because we assumed that the ascertainment of outcomes was not influenced by open (non‐blind) administration. Studies that met the inclusion criteria after screening were included in the review independent of their risk of bias classification. The assigned risk of bias in studies had an influence on the assessment of the certainty of the evidence (GRADE).
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients with hearing improvement measured by pure tone audiometry) as risk ratios (RR) with a 95% confidence interval (CI). For the key dichotomous outcomes presented in the summary of findings tables, we also expressed the results as absolute numbers (the assumed risk in the comparator group and the corresponding risk associated with the experimental intervention, based on its pooled relative effect and 95% CI).
For outcomes measured on a continuous scale (e.g. change in PTA, final PTA), we calculated the mean difference (MD) with a 95% CI. The summary statistic in the meta‐analysis for the primary outcome was the mean difference (MD) of the mean change in dB (baseline/post‐therapy) in hearing threshold between two groups in each study, measured by pure tone audiometry. The summary statistic in the meta‐analysis of the secondary outcome 'final hearing threshold' was the mean difference (MD) of the mean final hearing threshold in dB HL (post‐therapy) between two groups in each study, measured by pure tone audiometry. We used RevMan 5 to compute the measures of treatment effect for each individual study (RevMan 2020).
For hearing outcomes measured on a continuous scale (e.g. change in PTA, final PTA), we assumed a difference of 10 dB to be a clinically relevant effect. This decision was based on the test‐retest reliability of pure tone audiometric measurements, established minimal criteria for improvement in individual patients (Chandrasekhar 2019; Gurgel 2012; Stachler 2012), and on a large RCT on this topic with low risk of bias (Rauch 2011).
For dichotomous outcomes (e.g. proportion of patients with hearing improvement), we used a threshold of 25% or more in RR increase for appreciable benefit as suggested in the GRADE guideline (Guyatt 2011). The 10 dB difference and the 25% criteria were agreed upon by all authors.
Unit of analysis issues
The unit of analysis was the individual participant. We intended to include only studies in which the individual participant was the unit of analysis, regardless of whether they had unilateral or bilateral hearing loss. However, we did identify one study in which the unit of analysis was a single ear (Kosyakov 2011). As only a very small number of participants had bilateral hearing loss, we decided to include these data in the review. Although we were unable to account for the correlation between the ears, treating the data as independent is likely to produce a more conservative estimate of the treatment effect.
Dealing with missing data
We considered missing information about the methods of the included studies (e.g. when the method of randomisation was not reported) in the risk of bias assessment. Where data relating to an outcome of interest were not reported, we contacted the study authors. If the study authors could not provide the missing data or did not respond we excluded the study from the analysis of that outcome. If standard deviation data were not available, we approximated them using standard estimation methods from P values, standard errors or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2021).
Assessment of heterogeneity
We assessed both clinical and statistical heterogeneity. Clinical heterogeneity may be present even in the absence of statistical heterogeneity. For assessment of clinical heterogeneity we examined the included studies for evidence of major differences in the types of participants recruited, interventions, controls or outcomes measured.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test and the I² statistic. The latter calculates the percentage of variability that is not due to chance. I² values over 50% suggest the presence of substantial heterogeneity (Handbook 2021). Due to the low power of the Chi² test we set a significance level of P < 0.1.
Assessment of reporting biases
We assessed two aspects of reporting bias: between‐study publication bias and within‐study outcome reporting bias.
Publication bias (between‐study reporting bias)
Where sufficient studies (10 or more) were available for an outcome, we used a funnel plot to assess publication bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report with those listed in the methods section. If a study protocol was available, we compared the reported outcomes with the pre‐specified outcomes in the study protocol. When results were not reported in a statistically correct way this was reflected in a designation of high risk of bias due to selective outcome reporting.
Data synthesis
We used RevMan 5 to carry out meta‐analyses (RevMan 2020). Where possible we analysed data to give a summary measure of effect. We always used a fixed‐effect model for meta‐analysis to measure the effect. For dichotomous data, we analysed treatment differences as a risk ratio (RR). For continuous outcomes, if all the data were from the same scale, we pooled mean differences between values obtained at follow‐up and at baseline and reported this as a MD. We performed separate analyses for studies assessing primary and secondary therapy respectively.
Few studies reported the outcomes 'change in hearing threshold with speech audiometry' and 'frequency‐specific hearing loss'. Furthermore, studies often used different methods of speech audiometry and it was not clear if these were directly comparable. Therefore we have not conducted any meta‐analyses for these outcomes, but have instead shown the available data on a forest plot without pooling.
The type of adverse events varied widely between the different studies and it was often unclear whether these events had been systematically assessed and reported. Few studies reported an event rate for each randomised group. More often, a rate was reported for only one group, or a broad statement was made that 'no adverse events were observed'. Sometimes it was unclear to which group a statement applied. The lack of comparable data across groups and across studies meant we were unable to synthesise the data for many types of event and permitted few meta‐analyses.
We considered some adverse events to be directly related to the procedure of intratympanic injection, which may have explained why these events were not always assessed or reported in the comparator group. We considered people with ISSNHL to be very unlikely to experience tympanic membrane perforation, sudden‐onset vertigo (at the time of intratympanic injection) or sudden‐onset ear pain (at the time of intratympanic injection) unless directly attributable to the procedure. For these events we have provided a narrative synthesis of the event rate in the relevant group and presented these as a range.
For adverse events that could feasibly occur in either group, we have only included the studies that provided a rate for both groups, which allowed a comparison between the groups. A full description of all reported adverse event data is available for reference in Table 2, Table 4, Table 6 and Table 8.
Subgroup analysis and investigation of heterogeneity
We did not perform subgroup analysis, due to insufficient data for our planned analyses. We had planned to consider the following subgroups in the review:
Degree of hearing loss at initial presentation.
Age of patients.
Presence of vertigo and/or tinnitus.
Time before start of intratympanic treatment.
Duration of intratympanic treatment.
Drug delivery strategy/system used (e.g. intratympanic injection or continuous delivery etc).
Dose of intratympanic treatment.
Sensitivity analysis
We carried out sensitivity analyses to determine whether or not the findings were robust, based on the decisions made in undertaking the review. We planned analyses excluding studies with high risk of bias. Studies with high risk of bias were defined as those that had a high risk of selection bias (bias in randomisation or concealment, or both), an overall loss to follow‐up of > 25%, or unclear or imbalanced baseline parameters (e.g. treatment delay in Ashtiani 2018).
Summary of findings and assessment of the certainty of the evidence
Two independent authors (AL and CM) used the GRADE approach to rate the overall certainty of evidence. The certainty of evidence reflects the extent to which we are confident that an estimate of effect is correct and we considered this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high certainty of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low certainty implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency (heterogeneity);
indirectness of evidence (characteristics of participant population);
imprecision (variance of the outcome within studies); and
publication bias.
We included a summary of findings table, constructed according to the recommendations described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2021), for the following comparisons:
Intratympanic corticosteroids versus systemic corticosteroids as primary therapy.
Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as primary therapy.
Intratympanic corticosteroids versus no treatment or versus placebo as secondary therapy.
Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as secondary therapy.
We included the following outcomes in the summary of findings tables:
Change in hearing threshold with pure tone audiometry (pure tone average (PTA)).
Proportion of patients whose hearing is improved.
Final hearing threshold.
Adverse events.
As described above, adverse events were inconsistently reported across the studies, and a wide range of different adverse events were described. For the summary of findings tables, we therefore prioritised events that were considered to be of most relevance to intratympanic injection, namely tympanic membrane perforation, ear pain and vertigo/dizziness. All other adverse events are described in the text of the review and additional tables (Table 2; Table 4; Table 6; Table 8), but not included in the summary of findings tables.
The wording in the comments of the summary of findings tables, in the abstract, the results and the authors’ conclusion sections was based on the 'GRADE guidelines informative statements to communicate the findings of systematic reviews of interventions' (Santesso 2020). In this guideline, producers and users of systematic reviews found statements to communicate findings combining size and certainty of an effect acceptable. The Cochrane Handbook for Systematic Reviews of Interventions (Chapter 15.6.4) also suggests using these narrative statements for drawing conclusions based on the effect estimate from the meta‐analysis and the certainty of the evidence (Handbook 2021).
Results
Description of studies
Results of the search
The flow of records from the number of references identified in the search to the number of studies included in the review is shown in Figure 1. The database search yielded 1720 records after duplicates were removed. We identified 59 additional records through other sources. We screened 1779 records for initial inclusion and discarded 1399 records because they did not study intratympanic corticosteroid treatment of ISSNHL. We discarded a further 328 articles because they were reviews, case reports, study protocols or not randomised controlled studies (i.e. randomised controlled trials and quasi‐randomised controlled trials). Finally, we assessed 52 randomised and quasi‐randomised trials for eligibility. We excluded 20 studies because either the study was carried out in the wrong population (n = 3), or used the wrong intervention (n = 4) or wrong comparator (n = 12). We excluded one study because the duration of follow‐up was seven days or less. See Excluded studies and Characteristics of excluded studies.
1.
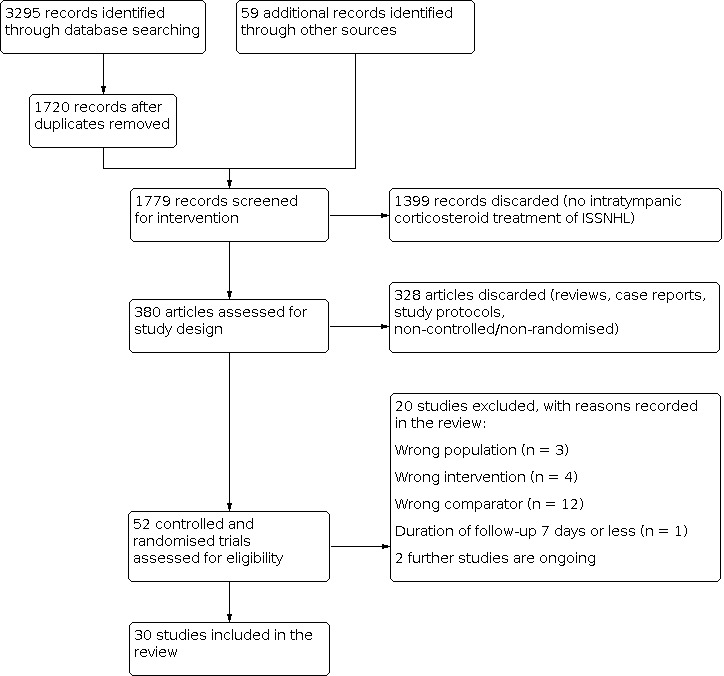
Process of selection of studies for inclusion in the review.
Two studies are ongoing. See Characteristics of ongoing studies.
We included the remaining 30 studies in the review.
Included studies
Thirty studies met the criteria for inclusion with 2133 analysed patients in total. See the Characteristics of included studies table for full details.
Study design
All included studies were parallel‐group RCTs. The majority of studies were open‐label trials. Only four studies reported blinding of participants, personnel and outcome assessors (Ashtiani 2018; Battaglia 2008; Plontke 2009; Wu 2011).
Participants
All included studies recruited adult participants. Studies were conducted in a number of locations, including China (Chang 2010; Huang 2021; Li 2011; Peng 2008; Qu 2015; Tong 2021; Wu 2011; Zhou 2011), the Republic of Korea (Ahn 2008; Choi 2011; Hong 2009; Lee 2011; Lim 2013), Greece (Koltsidopoulos 2013; Tsounis 2018; Xenellis 2006), Turkey (Arslan 2011; Ermutlu 2017; Gundogan 2013), Iran (Arastou 2013; Ashtiani 2018), the USA (Battaglia 2008; Rauch 2011), Germany (Plontke 2009), India (Swachia 2016), Italy (Dispenza 2011), Russia (Kosyakov 2011), Saudi Arabia (Al‐Shehri 2015), Sri Lanka (Rupasinghe 2017), and Taiwan (Ho 2004).
Baseline hearing loss
All participants had SSNHL, but the specific hearing threshold required by the studies did differ. The most common threshold was a hearing loss of > 30 dB in three contiguous frequencies, occurring over the course of < 72 hours (Ahn 2008; Choi 2011; Dispenza 2011; Ermutlu 2017; Gundogan 2013; Hong 2009; Huang 2021; Koltsidopoulos 2013; Kosyakov 2011; Lee 2011; Li 2011; Lim 2013; Swachia 2016; Tsounis 2018; Wu 2011; Xenellis 2006; Zhou 2011). Three studies did not describe the use of these thresholds in their methods, but defined SSNHL according to these criteria elsewhere in the article, therefore it is presumed that the same criteria were used (Arastou 2013; Ashtiani 2018; Peng 2008). Some studies used a smaller change in hearing threshold, such as > 20 dB hearing loss in three contiguous frequencies (Arslan 2011; Tong 2021), or > 10 dB hearing loss in three contiguous frequencies (Rupasinghe 2017). Again, one study did not describe the definition of SSNHL in the methods of the paper, but referred elsewhere to a definition of > 20 dB hearing loss in three contiguous frequencies, therefore we presumed this threshold was used (Battaglia 2008).
Two studies required participants to have a pure tone average of 50 dB or higher and the affected ear having hearing at least 30 dB worse than the contralateral (unaffected) ear (Al‐Shehri 2015; Rauch 2011). One study required a hearing threshold of ≥ 50 dB hearing loss for three or more frequencies (PTA including 0.5 kHz, 1 kHz, 2 kHz, 3 kHz and 4 kHz) or ≥ 60 dB for two frequencies, or ≥ 70 dB for any frequency within this range, or a speech reception threshold of ≥ 70 dB SPL or a speech discrimination score of ≥ 30% (Plontke 2009). Three studies did not provide a hearing threshold at which participants were included in the study (Chang 2010; Ho 2004; Qu 2015).
Time to initial treatment
For studies that were concerned with primary treatment, the majority enrolled and commenced treatment within 15 days of the onset of SSNHL (Al‐Shehri 2015; Ashtiani 2018; Choi 2011; Ermutlu 2017; Gundogan 2013; Hong 2009; Huang 2021; Qu 2015; Rauch 2011; Rupasinghe 2017; Swachia 2016; Tong 2021; Tsounis 2018). Five studies permitted enrolment in the study after a longer delay, but most participants were recruited within two weeks (Arslan 2011; Battaglia 2008; Dispenza 2011; Koltsidopoulos 2013; Kosyakov 2011). Four studies did not specify the time from onset of symptoms to treatment as an inclusion criterion. The delay to treatment in three of these studies was a mean of 7 days (Ahn 2008), 8.4 days (Lim 2013), and 5.6 days (Peng 2008). One study specifically recruited participants with poor prognostic factors, which may have included a delay in treatment (Arastou 2013). For this study, 38% of participants had a delay of more than two weeks before receiving their first treatment.
Failure of initial treatment
For studies that were concerned with secondary treatment, participants were recruited based on the failure of initial therapy. Treatment failure was also defined differently across the studies. Two studies based this purely on the improvement in hearing over the course of therapy, with improvement of < 10 dB (Lee 2011) or < 30 dB (Ho 2004) regarded as treatment failure. Three studies considered the difference between ears, with or without the absolute hearing threshold: Wu 2011 used a > 20 dB HL difference on PTA when compared to the contralateral (unaffected) ear to define treatment failure, whilst Li 2011 and Xenellis 2006 both used a > 10 dB HL difference when compared to the contralateral ear or a PTA of < 30 dB. Plontke 2009 required a hearing threshold in the contralateral ear to be at least 20 dB HL better than the affected ear in at least three frequencies between 0.5 kHz and 4 kHz. Zhou 2011 considered treatment failure as a change of less than 15 dB in PTA at four frequencies and an increase of < 15% in speech discrimination score after initial therapy. Chang 2010 stated that participants were included if they were refractory to primary therapy after 20 days, but did not describe how this was defined.
Interventions and comparisons
Most of the included studies investigated primary treatment of ISSNHL (Ahn 2008; Al‐Shehri 2015; Arastou 2013; Arslan 2011; Ashtiani 2018; Battaglia 2008; Choi 2011; Dispenza 2011; Ermutlu 2017; Gundogan 2013; Hong 2009; Huang 2021; Koltsidopoulos 2013; Kosyakov 2011; Lim 2013; Peng 2008; Qu 2015; Rauch 2011; Rupasinghe 2017; Swachia 2016; Tong 2021; Tsounis 2018).
For primary treatment, 12 studies compared intratympanic treatment to systemic steroids, which were predominantly administered orally (Al‐Shehri 2015; Dispenza 2011; Ermutlu 2017; Hong 2009; Huang 2021; Kosyakov 2011; Peng 2008; Qu 2015; Rauch 2011; Rupasinghe 2017; Swachia 2016; Tong 2021). Six studies compared combined treatment (intratympanic plus systemic corticosteroids) with systemic corticosteroids alone (Ahn 2008; Arastou 2013; Arslan 2011; Choi 2011; Gundogan 2013; Koltsidopoulos 2013). Four studies included three treatment arms (intratympanic treatment alone, intratympanic plus systemic treatment and systemic treatment alone) and therefore contributed data to both of these comparisons (Ashtiani 2018; Battaglia 2008; Lim 2013; Tsounis 2018).
A small number of studies investigated secondary treatment, after the failure of initial therapy (Chang 2010; Ho 2004; Lee 2011; Li 2011; Plontke 2009; Wu 2011; Xenellis 2006; Zhou 2011). The type of initial (primary) treatment that participants had received varied, with three studies using intravenous steroids (Plontke 2009; Xenellis 2006; Zhou 2011), three studies using a 10‐ to 14‐day course of oral steroids (Ho 2004; Lee 2011; Li 2011), and one study using an initial dose of intravenous steroids, followed by a tapered oral dose (Wu 2011). One study did not describe the primary therapy that had been used (Chang 2010).
Most studies that considered secondary treatment compared intratympanic steroids to either no treatment (Chang 2010; Ho 2004; Lee 2011; Li 2011; Xenellis 2006), or to placebo (Plontke 2009; Wu 2011). A single study compared intratympanic plus systemic corticosteroids to systemic corticosteroids alone (Zhou 2011).
The nature of the intratympanic injection varied between studies. Most studies used an intratympanic delivery of either dexamethasone or methylprednisolone, administered as a short course of three to four injections, typically over 7 to 14 days. Two studies used notably different methods of administration. One involved daily injections for 10 days, followed by alternate day injections for a further 20 days and ongoing injections twice a week for five months (Kosyakov 2011). One further study used a catheter to provide continuous infusion of dexamethasone over 14 days, rather than intermittent injections (Plontke 2009). In one study the duration of systemic treatment (15 days) was much shorter than that of the intratympanic treatment (six months) (Kosyakov 2011).
Outcomes
Duration of follow‐up varied across the studies. Four studies followed participants for 15 days or less (Arastou 2013; Arslan 2011; Plontke 2009; Qu 2015). Six studies followed participants for between 16 and 30 days (Ashtiani 2018; Chang 2010; Gundogan 2013; Lim 2013; Peng 2008; Wu 2011). The remaining studies followed participants for over one month, with a range of 38 days (Tong 2021) to 204 days (Dispenza 2011). Huang 2021 followed participants for 90 days, but we extracted data for change in hearing thresholds from an interim analysis at 12 days because the comparison of interest (intratympanic versus systemic corticosteroid) was administered only up to that point in time. All other outcome data were reported at the longest follow‐up point for each study, except instances where there was very high dropout at the final time point (as described in Included studies).
Change in hearing threshold with pure tone audiometry (pure tone average)
Most studies assessed hearing thresholds with a pure tone average based on four frequencies, either 0.5 kHz, 1 kHz, 2 kHz or 4 kHz (Al‐Shehri 2015; Arslan 2011; Choi 2011; Dispenza 2011; Kosyakov 2011; Li 2011; Peng 2008; Rauch 2011; Swachia 2016; Tsounis 2018; Wu 2011; Xenellis 2006; Zhou 2011), or 0.5 kHz, 1 kHz, 2 kHz and 3 kHz (Ahn 2008; Gundogan 2013; Hong 2009; Lee 2011; Lim 2013; Plontke 2009). Two studies used the average of three frequencies (0.5 kHz, 1 kHz, 2 kHz, Battaglia 2008; Ermutlu 2017), two studies used five frequencies (0.25 kHz, 0.5 kHz, 1 kHz, 2 kHz and 4kHz) (Arastou 2013; Ashtiani 2018), and four studies used six or more frequencies (Chang 2010; Ho 2004; Koltsidopoulos 2013; Tong 2021). Two studies did not describe the frequencies used (Qu 2015; Rupasinghe 2017).
Proportion of patients whose hearing is improved
We included data for this outcome regardless of the definition of 'improvement' used in the individual studies. However, this definition was not consistent across the different studies. A number of studies used the criterion of a specific change in hearing threshold to identify those who had improved. This was typically a change of at least 10 dB (Arslan 2011; Dispenza 2011; Ho 2004; Lee 2011; Li 2011; Lim 2013; Rauch 2011; Swachia 2016; Tong 2021; Wu 2011; Xenellis 2006), or 15 dB (Arastou 2013; Kosyakov 2011; Peng 2008; Qu 2015; Tsounis 2018; Zhou 2011), over the follow‐up period. Four studies used Siegel's criteria to assess improvement (Siegel 1975), where 'any' improvement is considered to be > 15 dB change in hearing threshold and final hearing threshold ≥ 75 dB (Ahn 2008; Choi 2011; Gundogan 2013; Hong 2009). Four studies considered both the change in hearing threshold and improvement in word recognition scores (WRS) or speech discrimination scores (SDS) when assessing improvement: Ashtiani 2018 (> 10 dB decrease in PTA or > 15% improvement in SDS), Battaglia 2008 (> 15 dB decrease in PTA or > 25% improvement in SDS), Ermutlu 2017 (> 10 dB decrease in PTA or > 10% improvement in WRS) and Koltsidopoulos 2013 (> 10 dB decrease in PTA and 15% improvement in SDS). One study used a decrease of > 30 dB in PTA, or an assessment of recovery to 50% of maximum possible (as compared to the unaffected ear) to indicate improvement (Plontke 2009). One study reported on improvement in hearing, but did not describe the criteria (Rupasinghe 2017). Two studies did not assess improvement (Al‐Shehri 2015; Chang 2010).
Final hearing threshold with pure tone audiometry
Frequencies used to assess this outcome were identical to those used for change in hearing threshold (see above).
Change in hearing threshold with speech audiometry
This outcome was only reported by a small number of studies, and was assessed with a variety of instruments, including speech discrimination scores, speech reception thresholds and word recognition scores. Considering the different metrics used to measure this outcome, and a concern that assessment conducted in different languages may not be directly comparable, we did not conduct any meta‐analyses. Six studies reported changes from baseline or final values for speech discrimination score (Ashtiani 2018; Battaglia 2008; Gundogan 2013; Koltsidopoulos 2013; Plontke 2009; Zhou 2011). Two studies reported changes from baseline in speech reception threshold (Ashtiani 2018; Plontke 2009), and one study reported change from baseline in the word recognition score (Rauch 2011).
Frequency‐specific changes with pure tone audiometry
Again, few studies reported on frequency‐specific changes with pure tone audiometry. There was also inconsistency in the frequencies that were assessed, and some studies presented pooled data across a small number of frequencies (low, mid and high), rather than reporting individual frequencies. Therefore we did not conduct any meta‐analyses for this outcome. The only studies assessing this were: Ahn 2008; Arslan 2011; Dispenza 2011; Gundogan 2013; Hong 2009; Huang 2021; Kosyakov 2011 Lee 2011; Lim 2013 and Tong 2021.
Mean level of improvement in those whose hearing is improved
This outcome was not assessed or reported by any of the included studies.
Percentage of patients reaching serviceable hearing (for those with profound pre‐treatment hearing loss)
This outcome was not assessed or reported by any of the included studies.
Effect on tinnitus and vertigo
This outcome was not assessed or reported by any of the included studies. Some studies reported on tinnitus and vertigo, but as adverse effects of the intervention, rather than assessing whether the intervention may have a beneficial effect on existing symptoms.
Minor and serious adverse effects
As described in Data synthesis, the adverse effects reported by the individual studies were wide‐ranging. The only adverse effects that were consistently reported across a large number of studies were those directly related to intratympanic injection (including persistent tympanic membrane perforation, pain or dizziness/vertigo at the time of the injection). However, as these events were clearly related to the intratympanic injection, and would not occur if participants received no treatment or systemic steroids, we considered it inappropriate to report a risk ratio comparing the intervention and comparator groups. Instead we have reported the rate of these complications for those individuals who received intratympanic injections. Specific details on other adverse events are included in the Effects of interventions and Table 2; Table 4; Table 6 and Table 8.
Excluded studies
See Characteristics of excluded studies.
Five randomised controlled trials compared the efficacy of intratympanic corticosteroid therapy in combination with hyperbaric oxygen therapy (HBO) (Attanasio 2015; Cho 2018; Gui‐li 2018; Sevil 2016; Zhou 2006), and one study used HBO treatment as a comparator (Cvorovic 2013). Since data suggest that HBO itself might have an effect on hearing recovery (Bennett 2012), and since the addition of HBO was not part of the interventions to be studied in this review (see methods), we excluded these studies.
Amizadeh 2021 compared combined corticosteroid treatment and systemic corticosteroid treatment as primary intervention. The study was excluded because the route of administration and dosage of systemic corticosteroid differed between groups.
The study Rogha 2017 and the trial registration NCT04766853 compared corticosteroid treatment by intratympanic injection of dexamethasone with intratympanic injection of dexamethasone mixed with hyaluronic acid. This type of comparison was not part of the review.
Chang 2020 compared intratympanic corticosteroid treatment with ear drop corticosteroid treatment as a primary intervention. This type of comparison was not part of the review.
Han 2021 compared intratympanic injection of corticosteroid versus corticosteroid administered via endoscopic tympanoplasty. This type of comparison was not part of the review.
We excluded the randomised controlled studies Berjis 2016 and Sun 2016 because two intratympanic treatment protocols using two different intratympanically applied corticosteroids were compared. This type of comparison was not part of the review.
We excluded Li 2016 because it compared intratympanic corticosteroid treatment with intratympanic corticosteroid plus mouse nerve growth factor treatment. This type of comparison was not part of the review.
Song 2018 compared intratympanic corticosteroid treatment with postauricular injection of corticosteroid as primary Intervention. This type of comparison was not part of the review.
In the study Park 2011, two methods of combination (intratympanic and systemic) therapy were compared. In the simultaneous intratympanic dexamethasone group, local drug application was given initially (as primary therapy for ISSNHL) with systemic steroids (intravenous dexamethasone followed by oral prednisolone). In the other "subsequent intratympanic dexamethasone group", intratympanic dexamethasone was given seven days after systemic treatment. There was no control group for the intratympanic salvage situation without local application. This type of comparison was not part of the review.
Filipo 2013 compared intratympanic corticosteroid treatment with intratympanic placebo as a primary intervention. The study endpoint was seven days after the start of treatment. Studies with a study endpoint of seven days or less after start of treatment were excluded from the review.
Choo 2017 compared intratympanic corticosteroid treatment, oral corticosteroid treatment and combined corticosteroid treatment as a primary intervention separated by low‐ or high‐frequency hearing loss. A comparison of hearing improvement in ISSNHL patients with low‐ and high‐frequency hearing loss was not part of the review.
We excluded Chen 2015 because the comparison group in this study included a mixture of patients receiving treatments with systemic steroids or systemic steroids plus intratympanic steroids.
We excluded Diao 2012 because the study population included a high proportion of patients with bilateral sudden hearing loss that raises doubt about whether they represented people with ISSNHL. Further, the unit of analysis in this study was ears instead of participants, as is used in this review.
Ongoing studies
Wang 2021 is a non‐blinded, parallel‐group randomised controlled trial that is being carried out in China, from October 2020. It compares nine intratympanic injections of dexamethasone over 14 days with daily oral prednisolone over 14 days for the primary treatment of ISSNHL. Pure tone thresholds, speech recognition, vestibular evoked myogenic potentials (VEMPs), Dizziness Handicap Inventory (DHI) and Tinnitus Handicap Inventory (THI) will be measured before treatment and one month after termination of treatment.
The study Yang 2020 is a non‐blinded, parallel‐group, randomised superiority trial that is being carried out in China, from January 2018. It compares four intratympanic injections of methylprednisolone over one week with daily intravenous methylprednisolone over five days for the primary treatment of ISSNHL in patients with diabetes mellitus. Pure tone thresholds will be measured before treatment and one month after termination of treatment. Secondary outcome measures will include the pure tone average at three months after treatment and blood glucose changes during treatment.
Risk of bias in included studies
We deemed the risk of bias to be generally rather high in most of the included studies. We assigned only four of the 30 included studies an overall low risk of bias (Plontke 2009; Rauch 2011; Tsounis 2018; Wu 2011). An overview of the risk of bias for each included study is provided in Figure 2. Figure 3 presents the proportion of each risk of bias domain that we found to be high risk/low risk/unclear risk across the whole review.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Randomisation was adequate in nine studies (Arastou 2013; Ashtiani 2018; Gundogan 2013; Kosyakov 2011; Plontke 2009; Qu 2015; Tsounis 2018; Rauch 2011; Wu 2011). Ahn 2008 also used computerised random allocation, but patients who refused the allocated therapy were excluded. Twelve studies stated that they were randomised without details concerning the methods of randomisation (Al‐Shehri 2015; Battaglia 2008; Chang 2010; Choi 2011; Dispenza 2011; Ermutlu 2017; Ho 2004; Hong 2009; Lee 2011; Li 2011; Swachia 2016; Xenellis 2006). Eight studies used inadequate randomisation methods (randomisation according to sequence of admission or actual date) (Arslan 2011; Huang 2021; Koltsidopoulos 2013; Lim 2013; Peng 2008; Rupasinghe 2017; Tong 2021; Zhou 2011).
Allocation concealment was adequate in only five studies (Ashtiani 2018; Plontke 2009; Rauch 2011; Tsounis 2018; Wu 2011). The other studies reported either an inadequate method of concealment or it was not mentioned at all. In studies that used sequence of admission or actual date for randomisation adequate allocation concealment is not possible (Arslan 2011; Huang 2021; Koltsidopoulos 2013; Lim 2013; Peng 2008; Rupasinghe 2017; Tong 2021; Zhou 2011).
Blinding
Only four studies used placebo therapy with blinding of participants and personnel during their trials (Ashtiani 2018; Battaglia 2008; Plontke 2009; Wu 2011). Twenty‐six studies were not placebo‐controlled. In one, it was explicitly stated that participants and personnel were not blinded (Tsounis 2018); in the others we assumed that neither were blinded. We judged the risk of bias in non‐placebo‐controlled studies to be generally 'low' assuming that the outcomes were not influenced by open administration of study therapy. In two studies patients in the intratympanic treatment group could refuse the therapy after allocation, so we deemed the risk of bias to be high (Ahn 2008; Zhou 2011).
In 13 studies, we considered the risk of detection bias to be low because they were either placebo‐controlled, or there was blinding of outcome assessment (Al‐Shehri 2015; Arastou 2013; Ashtiani 2018; Battaglia 2008; Hong 2009; Koltsidopoulos 2013; Kosyakov 2011; Lim 2013; Rauch 2011; Plontke 2009; Tong 2021; Tsounis 2018; Wu 2011). Seventeen studies gave no information on blinding of outcome assessment and the risk of bias was unclear.
Incomplete outcome data
Fourteen studies reported results for all randomised participants. The study Rauch 2011 reported a low dropout rate below 5%. We classified six studies as high risk of bias because the dropout rate was higher than 10% (Ashtiani 2018; Battaglia 2008; Ermutlu 2017; Hong 2009; Rupasinghe 2017; Tsounis 2018), and we classified three studies as high risk because dropouts were not balanced across treatment arms (Dispenza 2011) or were related to the therapy (Wu 2011; Zhou 2011). The studies Huang 2021, Gundogan 2013, Plontke 2009 and Tong 2021 reported a moderate rate of dropout (5% to 10%) but it was balanced across treatment arms. Ahn 2008 and Chang 2010 gave insufficient information to permit judgement as they did not report the number of participants that were analysed.
Selective reporting
Twenty‐one studies specified and reported the main outcome measures (Ahn 2008; Al‐Shehri 2015; Arslan 2011; Ashtiani 2018; Chang 2010; Ermutlu 2017; Gundogan 2013; Hong 2009; Huang 2021; Kosyakov 2011; Li 2011; Lim 2013; Peng 2008; Plontke 2009; Qu 2015; Rauch 2011; Swachia 2016; Tong 2021; Tsounis 2018; Wu 2011; Xenellis 2006). There was no indication of selective reporting in these studies. Four studies failed to report the standard deviation for change in hearing threshold (Arastou 2013; Battaglia 2008; Choi 2011; Lee 2011). Some studies included several follow‐up time points but results were only shown for the last time point (Choi 2011; Dispenza 2011; Ho 2004; Lee 2011; Zhou 2011). In two studies contradictions were present between pre‐specified outcome parameters in the methods section and the presented outcomes in the results: in Koltsidopoulos 2013 a 7PTA was defined as the primary outcome parameter but the reported hearing loss before treatment and hearing improvement were both based on a 4PTA. In the study Tong 2021, a decrease in PTA of > 30 dB HL was a criterion for successful treatment, but a > 10 dB HL decrease was actually used. In Rupasinghe 2017, the criteria for hearing improvement were not reported.
Other potential sources of bias
A common source of bias was imbalance between groups for a number of factors: there were often unexplained differences in the number of participants in each group (Arastou 2013; Arslan 2011; Choi 2011; Lee 2011; Li 2011; Qu 2015; Swachia 2016), and differences between groups in the delay before commencing treatment (Battaglia 2008; Dispenza 2011; Lim 2013; Rupasinghe 2017; Xenellis 2006). In two studies there was a difference between groups in the length of treatment (Kosyakov 2011; Peng 2008). In one study, follow‐up was longer in the intervention group than in the comparator group (Lim 2013). Only the studies Ashtiani 2018, Koltsidopoulos 2013, Lim 2013, Plontke 2009 and Tsounis 2018 performed a sample size determination. Other studies either did not do so (Ahn 2008; Al‐Shehri 2015; Arastou 2013; Arslan 2011; Chang 2010; Choi 2011; Dispenza 2011; Ermutlu 2017; Gundogan 2013; Ho 2004; Hong 2009; Huang 2021; Kosyakov 2011; Lee 2011; Li 2011; Peng 2008; Qu 2015; Rupasinghe 2017; Swachia 2016; Tong 2021; Wu 2011; Xenellis 2006; Zhou 2011), or terminated recruitment before reaching a sufficient number of participants based on sample size calculation (Battaglia 2008). In consequence, the number of included participants per treatment arm was small in most studies. Studies without sample size determination are prone to type II errors. This is not discussed in any of these studies. Small study populations are also prone to imbalances between treatment arms in terms of potential confounding factors, including the propensity in some patients with ISSNHL towards spontaneous hearing recovery.
A broad range of delay between the onset of symptoms and the start of treatment was evident in some studies (Arastou 2013; Arslan 2011; Battaglia 2008; Peng 2008; Xenellis 2006). Treatment delay is recognised as one of the main factors that influences the observed hearing improvement (Liebau 2017). When there is a small number of participants per group, studies may differ in this respect across treatment arms. As noted above, a noticeable difference in treatment delay was evident in a number of studies (Battaglia 2008; Dispenza 2011; Lim 2013; Rupasinghe 2017; Xenellis 2006). This could have influenced the reported difference in outcome between treatment arms.
The main baseline parameter that influences the observed hearing improvement is the level of hearing loss of the patient at the beginning of the observation period (Liebau 2017). Although a balanced hearing loss before treatment between intervention arms is extremely important, in two studies hearing loss before treatment differed by more than 10 dB HL across groups (Choi 2011; Dispenza 2011). The differences in hearing loss before treatment between the intervention arms may have influenced the reported difference in outcome.
Many studies did not report the baseline characteristics of their treatment arms, or reported them inadequately. In 13 studies the treatment delay in each treatment arm was not reported (Al‐Shehri 2015; Ashtiani 2018; Chang 2010; Ho 2004; Kosyakov 2011; Lee 2011; Li 2011; Qu 2015; Rupasinghe 2017; Swachia 2016; Wu 2011; Xenellis 2006; Zhou 2011), or a standard deviation is missing in that parameter (Dispenza 2011; Hong 2009). In the study Rupasinghe 2017, the hearing thresholds before treatment in participants per group are not reported and Dispenza 2011 omitted the standard deviation for that parameter. The studies Zhou 2011 and Ho 2004 reported the mean hearing loss before treatment in the intervention group but not in the control group.
The studies Arslan 2011, Plontke 2009, Qu 2015 and Zhou 2011 conducted a very short follow‐up (two weeks or less), which could result in a bias in the estimation of treatment effects. In Huang 2021, the comparison of interest for this review (intratympanic corticosteroid versus systemic corticosteroid) was observed for only 12 days from the start of treatment, the comparator group receiving systemic treatment then switching to intratympanic corticosteroid. As noted above, in Lim 2013 the duration of follow‐up was longer in the intervention group in comparison to the control group (21 versus 17 days). This could underestimate the treatment effect in the control group. In Peng 2008, the duration of treatment differed between treatment arms (17 versus 27 days). This was also true of Kosyakov 2011 (six months versus 15 days). In each case, the discrepancy in the duration of treatment could bias the estimate of effect. Rupasinghe 2017 included patients with very mild hearing loss (> 10 dB HL) and Kosyakov 2011 included only mild cases of ISSNHL. By contrast, Zhou 2011 included only patients with poor prognosis (see the inclusion criteria of the study for details). All three studies risk bias due to selection of the study population. In Xenellis 2006, the omission of overall hearing improvement in the control group is unlike the other studies but is not discussed by the authors.
Effects of interventions
See: Table 1; Table 3; Table 5; Table 7
Intratympanic corticosteroids versus no treatment or versus placebo as primary therapy
No study compared the effects of intratympanic corticosteroids versus no treatment or placebo on hearing improvement for primary therapy of ISSNHL.
Intratympanic corticosteroids versus systemic corticosteroids as primary therapy
Sixteen studies compared the efficacy of a primary intratympanic corticosteroid treatment with systemic corticosteroid treatment (Al‐Shehri 2015; Ashtiani 2018; Battaglia 2008; Dispenza 2011; Ermutlu 2017; Hong 2009; Huang 2021; Kosyakov 2011; Lim 2013; Peng 2008; Qu 2015; Rauch 2011; Rupasinghe 2017; Swachia 2016; Tong 2021; Tsounis 2018).
Change in hearing threshold with pure tone audiometry (pure tone average)
Ten studies (701 participants) were included in the meta‐analysis. Battaglia 2008 could not be included as no variance was reported. The mean change in PTA between baseline and 17 to 182 days (range) after start of therapy in participants with intratympanic therapy was ‐5.93 dB (95% confidence interval (CI) ‐7.61 to ‐4.26; 701 participants; 10 studies; I2 = 80%; low‐certainty evidence) (Analysis 1.1). The point estimate of effect did not exceed the minimally important difference of ‐10 dB, however. Primary intratympanic therapy may result, therefore, in little to no improvement in hearing threshold compared to systemic steroids.
1.1. Analysis.

Comparison 1: Intratympanic compared to systemic corticosteroids as primary therapy, Outcome 1: Mean change in pure tone average (PTA)
Although we noted high heterogeneity, we considered this unlikely to affect the conclusion of the analysis, as most studies resulted in an estimated effect size that was of borderline clinical significance (did not exceed the minimally important difference). Rauch 2011 and Tsounis 2018 were included in the pre‐planned sensitivity analysis. These two studies (319 participants) found that the mean change of PTA in participants with intratympanic treatment was lower compared with the systemic treatment group but did not exceed the minimally important difference of 10 dB (mean difference (MD) 2.00, 95% CI ‐2.79 to 6.79; 319 participants; 2 studies; I2 = 0%). The sensitivity analysis therefore confirmed the result from our primary analysis. As the treatment regime for Kosyakov 2011 was extremely different from all other studies (six months of intratympanic corticosteroid), and as the duration of treatment differed so markedly between treatment arms (the comparator group receiving only 15 days of systemic corticosteroid), we conducted an additional sensitivity analysis to investigate the degree to which excluding this study would impact the pooled estimate. After its exclusion, the pooled mean difference again only indicated a trivial effect in favour of intratympanic therapy (MD ‐2.81, 95% CI ‐4.49 to ‐0.66; 651 participants; 9 studies; I2 =67%; low‐certainty evidence). We concluded that the inclusion of this study in the meta‐analysis did not affect the pooled effect estimate to any serious degree.
Proportion of patients whose hearing is improved
Fourteen studies (972 participants) were included in the meta‐analysis. In one study there was high (35.1%) loss to follow‐up at the final (three‐month) assessment (Rupasinghe 2017). We therefore extracted outcome data from the penultimate assessment at one month. A slightly higher proportion of participants with intratympanic therapy had improved hearing at 10 to 182 days (range) after the start of treatment compared with the systemic treatment group. The point estimate of effect did not, however, exceed the minimally important difference of 25% and the 95% confidence interval included no difference between groups (risk ratio (RR) 1.04, 95% CI 0.97 to 1.12; 972 participants; 14 studies; I2 = 16%) (Analysis 1.2). Primary intratympanic therapy, therefore, probably results in little to no difference in the proportion of patients whose hearing is improved compared to systemic corticosteroids.
1.2. Analysis.

Comparison 1: Intratympanic compared to systemic corticosteroids as primary therapy, Outcome 2: Proportion improved
The studies Ashtiani 2018, Rauch 2011 and Tsounis 2018 were included in the sensitivity analysis. The three studies (396 participants) found that fewer participants with intratympanic treatment had improvement of hearing compared with the systemic treatment group but the difference did not exceed the minimally important difference of 25% (RR 0.96, 95% CI 0.86 to 1.07; 396 participants; 3 studies; I2 = 16%). The sensitivity analysis therefore confirmed the result from our primary analysis.
Final hearing threshold with pure tone audiometry (pure tone average)
Seven studies (516 participants) were included in the meta‐analysis. The study Tsounis 2018 could not be included as no variance was reported. The final PTA at 17 to 183 days (range) after the start of therapy in participants with intratympanic therapy was lower (better) compared with the systemic treatment group. The point estimate of effect did not, however, exceed the minimally important difference of 10 dB HL (MD ‐3.31, 95% CI ‐6.16 to ‐0.47; 516 participants; 7 studies; I2 = 41%; low‐certainty evidence) (Analysis 1.3). Primary intratympanic therapy may result, therefore, in little to no difference in the final hearing threshold compared to systemic corticosteroids. The study Rauch 2011 was included in the sensitivity analysis and confirmed this result (MD 1.60, 95% CI ‐5.75 to 8.95; 250 participants; 1 study).
1.3. Analysis.

Comparison 1: Intratympanic compared to systemic corticosteroids as primary therapy, Outcome 3: Final PTA
Change in hearing threshold with speech audiometry
Only one study reported on the change in hearing threshold with speech audiometry (Ashtiani 2018). The speech reception threshold may be lower (better) in the group who received intratympanic corticosteroids compared to those who received systemic corticosteroids, although the confidence interval crosses unity (MD ‐8.85 dB, 95% CI ‐19.58 to 1.88; 98 participants; 1 study) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Intratympanic compared to systemic corticosteroids as primary therapy, Outcome 4: Change in hearing threshold with speech audiometry
Three further studies used methods other than hearing threshold to assess speech audiometry (Ashtiani 2018; Battaglia 2008; Rauch 2011). These studies reported on the change in speech recognition or discrimination, using either a speech discrimination score (SDS) or a word recognition score (WRS). Although these did not relate to our pre‐specified outcome (change in hearing threshold with speech audiometry) we considered that they were assessing the same underlying outcome (speech audiometry), therefore we have included them for completeness. Due to the different assessment tools used, inconsistency in terminology (making it unclear whether measures were comparable), and the different languages in which these were conducted, we considered that it was not appropriate to pool the data. The results of these additional measures are presented in Analysis 1.5.
1.5. Analysis.

Comparison 1: Intratympanic compared to systemic corticosteroids as primary therapy, Outcome 5: Speech audiometry: additional outcomes
The study Ermutlu 2017 performed speech audiometry (speech recognition threshold, word recognition score) but did not report results separately for these tests ‐ they were instead reported as part of a composite outcome for 'recovery'. Therefore this study was not included for this outcome.
Frequency‐specific changes with pure tone audiometry
Five studies reported on frequency‐specific changes with pure tone audiometry (Dispenza 2011; Hong 2009; Huang 2021; Lim 2013; Tong 2021). Due to the different frequencies assessed in the studies, and heterogeneity in the effect estimates where studies did assess the same frequency, we did not meta‐analyse these data. Instead the results from each study are shown in Analysis 1.6.
1.6. Analysis.

Comparison 1: Intratympanic compared to systemic corticosteroids as primary therapy, Outcome 6: Frequency‐specific changes in PTA
Mean level of improvement, in those whose hearing is improved
This outcome was not reported by any of the included studies.
Percentage of patients reaching serviceable hearing
This outcome was not reported by any of the included studies.
Effect on tinnitus and vertigo
This outcome was not reported by any of the included studies.
Minor and serious adverse events
For this comparison, 12 studies provided information about adverse events (Al‐Shehri 2015; Dispenza 2011; Ermutlu 2017; Hong 2009; Huang 2021; Kosyakov 2011; Qu 2015; Rauch 2011; Rupasinghe 2017; Swachia 2016; Tong 2021; Tsounis 2018). In four studies, reporting was incomplete, either because a rate was not provided for both randomised groups (Kosyakov 2011; Swachia 2016), or because it was unclear in which group (or groups) events were observed (Qu 2015; Tsounis 2018). In one additional study adverse event data were not reported specifically for the period of follow‐up during which the allocated interventions matched the comparison of interest for this review (Huang 2021). Despite these limitations in the reporting of adverse events, meta‐analysis was possible for some adverse event outcomes (Analysis 1.7).
1.7. Analysis.

Comparison 1: Intratympanic compared to systemic corticosteroids as primary therapy, Outcome 7: Adverse events
Persistent tympanic membrane perforation
Four studies reported a rate of tympanic membrane perforation of between 0% (0/30) and 3.9% (5/129) for those who received an intratympanic corticosteroid injection (Huang 2021; Kosyakov 2011; Rauch 2011; Tong 2021). Note that in one study both groups received intratympanic injection: one group received intratympanic corticosteroid, and the other received intravenous followed by intratympanic corticosteroid (Huang 2021). We concluded that the evidence is very uncertain regarding the risk of tympanic membrane perforation for those who received intratympanic corticosteroid as primary treatment (463 participants; 4 studies; very low‐certainty evidence).
Vertigo/dizziness, timing not reported
A single study provided a comparison between intratympanic and systemic corticosteroid, resulting in a risk ratio of 2.53 (95% CI 1.41 to 4.54) (Rauch 2011). It is not specified whether all of the patients in the intratympanic corticosteroid group experiencing vertigo did so at the time of injection. We concluded that intratympanic therapy may increase the risk of vertigo/dizziness of unspecified timing as compared to systemic corticosteroid (250 participants; 1 study; low‐certainty evidence).
Vertigo/dizziness at the time of intratympanic injection
Four studies reported a rate of vertigo/dizziness of between 1.5% (1/67) and 21% (4/19) for those who received an intratympanic injection (Ermutlu 2017; Huang 2021; Tong 2021; Tsounis 2018). We have included in this analysis all participants who received an intratympanic injection in these studies. For two studies, this included participants in another treatment arm. In Huang 2021, participants in the control arm also received intratympanic injection at a later point in the trial. In Tsounis 2018, data were reported for participants who received intratympanic corticosteroids alone, and combined therapy with intratympanic corticosteroids and systemic corticosteroids. We concluded that the evidence is very uncertain regarding the risk of vertigo/dizziness at the time of intratympanic corticosteroid treatment as primary therapy (301 participants; 4 studies; very low‐certainty evidence).
Ear pain, timing not reported
Two studies contributed data to a meta‐analysis (Al‐Shehri 2015; Rauch 2011). The risk ratio was 15.68 (95% CI 6.22 to 39.49), favouring systemic corticosteroid. In each study, the number of participants with ear pain/earache was presented separately from the numbers with ear pain at intratympanic injection. It was assumed, therefore, that those participants with pain at injection were not included among those with ear pain/earache. We concluded that intratympanic corticosteroid injection probably increases the risk of ear pain as compared to systemic corticosteroid when used as primary treatment (289 participants; 2 studies; moderate‐certainty evidence).
Ear pain at the time of injection
Three studies reported a rate of ear pain from 4.8% (5/104) to 27.1% (35/129) (Al‐Shehri 2015; Huang 2021; Rauch 2011). In Al‐Shehri 2015 and Rauch 2011, the number of participants with ear pain/earache was presented separately from the numbers with ear pain at intratympanic injection. It was assumed, therefore, that those participants with pain at injection were not included among those with ear pain/earache. The evidence suggests that there is a risk of ear pain at the time of intratympanic injection of corticosteroid as primary treatment (393 participants; 3 studies; low‐certainty evidence).
Mood change
Two studies contributed data to a meta‐analysis (Al‐Shehri 2015; Rauch 2011). The risk ratio was 0.22 (95% CI 0.13 to 0.37), favouring intratympanic corticosteroid. We concluded that intratympanic corticosteroid likely results in a large reduction in risk compared to systemic corticosteroids (289 participants; 2 studies; moderate‐certainty evidence).
Blood glucose problems
Two studies contributed data to a meta‐analysis (Al‐Shehri 2015; Rauch 2011). The risk ratio was 0.54 (95% CI 0.35 to 0.85), favouring intratympanic corticosteroid. We concluded that intratympanic corticosteroid may result in a reduction in risk compared to systemic corticosteroid (289 participants; 2 studies; low‐certainty evidence).
Sleep change
Two studies contributed data to a meta‐analysis (Al‐Shehri 2015; Rauch 2011). The risk ratio was 0.19 (95% CI 0.10 to 0.36), favouring intratympanic corticosteroid. We concluded that intratympanic corticosteroid likely results in a large reduction in risk compared to systemic corticosteroid (289 participants; 2 studies; moderate‐certainty evidence).
Appetite change
Two studies contributed data to a meta‐analysis (Al‐Shehri 2015; Rauch 2011). The risk ratio was 0.20 (95% CI 0.09 to 0.44), favouring intratympanic corticosteroid. We concluded that intratympanic corticosteroid likely results in a large reduction in risk compared to systemic corticosteroid (289 participants; 2 studies; moderate‐certainty evidence).
Weight change
Two studies contributed data to a meta‐analysis (Al‐Shehri 2015; Rauch 2011). The risk ratio was 0.28 (95% CI 0.13 to 0.61), favouring intratympanic corticosteroid. We concluded that intratympanic corticosteroid likely results in a large reduction in risk compared to systemic corticosteroid (289 participants; 2 studies; moderate‐certainty evidence).
Dry mouth
Two studies contributed data to a meta‐analysis (Al‐Shehri 2015; Rauch 2011). The risk ratio was 0.15 (95% CI 0.06 to 0.35), favouring intratympanic corticosteroid. We concluded that intratympanic corticosteroid likely results in a large reduction in risk compared with systemic corticosteroids (289 participants; 2 studies; moderate‐certainty evidence).
Otitis media
One study reported a rate for each group (Rauch 2011). The risk ratio was 3.28 (95% CI 0.70 to 15.49), favouring systemic corticosteroid. We concluded that intratympanic corticosteroid may result in a large increase in risk compared to systemic corticosteroid (250 participants; 1 study; low‐certainty evidence).
Table 2 provides details of the more limited data on other reported adverse events.
Intratympanic plus systemic corticosteroids (combined therapy) versus no treatment or versus placebo as primary therapy
No study compared the effects of intratympanic corticosteroids plus systemic corticosteroids (combined therapy) versus no treatment or versus placebo on hearing improvement for primary therapy of ISSNHL.
Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as primary therapy
Ten studies compared the efficacy of a primary combined therapy with a systemic corticosteroid therapy (Ahn 2008; Arastou 2013; Arslan 2011; Ashtiani 2018; Battaglia 2008; Choi 2011; Gundogan 2013; Koltsidopoulos 2013; Lim 2013; Tsounis 2018). Three of our secondary outcome measures were not reported by any of the included studies.
Change in hearing threshold with pure tone audiometry (pure tone average)
Six studies (435 participants) were included in the meta‐analysis. The study Battaglia 2008 could not be included as no variance was reported. The mean change in PTA between baseline and 15 to 91 days (range) after start of therapy improved more in participants with combined therapy. The point estimate of effect did not exceed the minimally important difference of ‐10 dB (MD ‐8.55, 95% CI ‐12.48 to ‐4.61; 435 participants; 6 studies; I2 = 32%; low‐certainty evidence) (Analysis 2.1). Primary combined therapy may result, therefore, in a slight improvement in hearing threshold compared to systemic corticosteroids alone, but it is not certain whether the extent of improvement would be meaningful to people with ISSNHL. The study Tsounis 2018 was included in the sensitivity analysis and confirmed this result (MD 0.80, 95% CI ‐8.41 to 10.01; 68 participants; 1 study).
2.1. Analysis.

Comparison 2: Combined compared to systemic corticosteroids as primary therapy, Outcome 1: Mean change in pure tone average (PTA)
Proportion of patients whose hearing is improved
Ten studies (788 participants) were included in the meta‐analysis. A higher proportion of participants with combined therapy had improvement of hearing at 15 to 91 days (range) after start of treatment. The point estimate of effect exceeded the minimally important difference of 25% (RR 1.27, 95% CI 1.15 to 1.41; 788 participants; 10 studies; I2 = 47%) (Analysis 2.2). Primary combined therapy may, therefore, increase the proportion of patients whose hearing is improved compared to systemic corticosteroids alone (low‐certainty evidence). The studies Ashtiani 2018 and Tsounis 2018 were included in the sensitivity analysis and found that more participants with combined therapy had improvement of hearing but this did not exceed the minimally important difference of 25% (RR 1.08, 95% CI 0.88 to 1.33; 148 participants; 2 studies; I2 = 0%).
2.2. Analysis.

Comparison 2: Combined compared to systemic corticosteroids as primary therapy, Outcome 2: Proportion improved
Final hearing threshold with pure tone audiometry (pure tone average)
Three studies (194 participants) were included in the meta‐analysis. The studies Arastou 2013 and Tsounis 2018 could not be included since no variance was reported. The final PTA at 15 to 56 days (range) after start of therapy in participants with combined therapy was lower (more favourable) when compared with the systemic treatment group but the point estimate of effect did not exceed the minimally important difference of ‐10 dB (MD ‐9.11, 95% CI ‐1.67 to ‐16.56; 194 participants; 3 studies; I2 = 35%; very low‐certainty evidence) (Analysis 2.3). Primary combined therapy may result, therefore, in slightly lower (better) final hearing thresholds compared to systemic corticosteroids alone, but the evidence is very uncertain. No eligible studies could be identified for a sensitivity analysis.
2.3. Analysis.

Comparison 2: Combined compared to systemic corticosteroids as primary therapy, Outcome 3: Final PTA
Change in hearing threshold with speech audiometry
One study reported on the change in speech recognition threshold (Ashtiani 2018). The speech reception threshold may be lower (better) in the group who received combination treatment compared to those who received systemic corticosteroids, although the confidence interval crosses unity (mean difference ‐7.59 dB; 95% CI ‐20.22 to 5.04; 98 participants; 1 study) (Analysis 2.4).
2.4. Analysis.

Comparison 2: Combined compared to systemic corticosteroids as primary therapy, Outcome 4: Change in hearing threshold with speech audiometry
Four studies reported on alternative measures of speech audiometry (Ashtiani 2018; Battaglia 2008; Gundogan 2013; Koltsidopoulos 2013). The results of these additional measures are presented in Analysis 2.5. All had an effect direction that favoured combined treatment. Koltsidopoulos 2013 reported on the change in speech discrimination score using medians and an interquartile range, therefore these data are not portrayed in Analysis 2.5. The authors reported that the results favoured combined therapy, but the difference was not statistically significant (median change in combined group 32% (interquartile range (IQR) 8.5 to 60.5%), median change in systemic group 18% (IQR 2.0 to 50.5%)).
2.5. Analysis.

Comparison 2: Combined compared to systemic corticosteroids as primary therapy, Outcome 5: Speech audiometry: additional outcomes
Frequency‐specific changes with pure tone audiometry
Four studies assessed changes in hearing level at specific frequencies (Ahn 2008; Arslan 2011; Gundogan 2013; Lim 2013). Due to the different frequencies assessed in the studies, and heterogeneity in the effect estimates where studies did assess the same frequency, we did not meta‐analyse these data. Instead the results from each study are shown in Analysis 2.6.
2.6. Analysis.

Comparison 2: Combined compared to systemic corticosteroids as primary therapy, Outcome 6: Frequency‐specific changes with PTA
Mean level of improvement, in those whose hearing is improved
This outcome was not reported by any of the included studies.
Percentage of patients reaching serviceable hearing
This outcome was not reported by any of the included studies.
Effect on tinnitus and vertigo
This outcome was not reported by any of the included studies.
Minor and serious adverse events
For this comparison, eight studies provided information pertaining to adverse events (Ahn 2008; Arastou 2013; Arslan 2011; Battaglia 2008; Choi 2011; Gundogan 2013; Koltsidopoulos 2013; Tsounis 2018). In three studies reporting was incomplete for one or more adverse event outcomes, because it was unclear in which group (or groups) events were observed (Arastou 2013; Arslan 2011; Tsounis 2018). There were insufficient data for meta‐analysis.
Persistent tympanic membrane perforation
Five studies reported a rate of perforation between 0% (0/85) and 5.5% (2/36) for those who received an intratympanic injection (Ahn 2008; Arastou 2013; Arslan 2011; Choi 2011; Gundogan 2013). We concluded that the evidence is very uncertain regarding the risk of tympanic membrane perforation for those who received intratympanic corticosteroid combined with systemic corticosteroid as primary treatment (474 participants; 5 studies; very low‐certainty evidence).
Vertigo/dizziness at the time of injection
Four studies reported a rate between 0% (0/60) and 8.1% (3/37) for those who received an intratympanic injection (Ahn 2008; Choi 2011; Gundogan 2013; Tsounis 2018). Note that in one study, two groups received intratympanic injection: one group received intratympanic corticosteroid, and the other received intratympanic and systemic corticosteroid (Tsounis 2018). We concluded that the evidence is very uncertain regarding the risk of vertigo/dizziness at the time of intratympanic injection for those who received intratympanic corticosteroid as primary treatment (341 participants; 4 studies; very low‐certainty evidence).
Ear pain at the time of injection
One study reported a rate of 5/37 (13.5%) for those who received an intratympanic corticosteroid injection (Gundogan 2013). All recovered within one hour. We concluded that the evidence is very uncertain regarding the risk of ear pain at the time of intratympanic injection for those who received combined treatment as primary treatment (73 participants; 1 study; very low‐certainty evidence).
Table 4 provides details of the more limited data on other reported adverse events.
Intratympanic corticosteroids versus no treatment or versus placebo as secondary therapy
There were five studies comparing the efficacy of a secondary intratympanic corticosteroid with no therapy (Chang 2010; Ho 2004; Lee 2011; Li 2011; Xenellis 2006), and two studies comparing intratympanic corticosteroid with intratympanic placebo (Plontke 2009; Wu 2011).
Change in hearing threshold with pure tone audiometry (pure tone average)
Seven studies (280 participants) were included in the meta‐analysis. The mean change in PTA between baseline and 20 to 60 days (range) after the start of therapy showed more improvement in participants with intratympanic treatment but the point estimate of effect did not exceed the minimally important difference of ‐10 dB (MD ‐9.07 dB, 95% CI ‐11.47 to ‐6.66; 280 participants; 7 studies; I2 = 23%; low‐certainty evidence) (Analysis 3.1). Secondary (rescue) intratympanic therapy may therefore result in a small benefit compared to no treatment or placebo, but it is not clear whether this would be important to patients. The studies Plontke 2009 and Wu 2011 were included in a sensitivity analysis and confirmed the result of our primary analysis (MD ‐5.45 dB, 95% CI ‐9.30 to ‐1.59; 76 participants; 2 studies; I2 = 0%).
3.1. Analysis.

Comparison 3: Intratympanic corticosteroids compared to no treatment/placebo as secondary therapy, Outcome 1: Mean change in PTA
Proportion of patients whose hearing is improved
Six studies (232 participants) were included in the meta‐analysis. The study Chang 2010 could not be included since this parameter was not reported. A higher proportion of participants with intratympanic therapy had improved hearing at 29 to 79 days (range) after the start of treatment (RR 5.55, 95% CI 2.89 to 10.68; 232 participants; 6 studies; I2 = 0%; low‐certainty evidence) (Analysis 3.2). Secondary (rescue) intratympanic therapy may therefore result in a much higher proportion of patients whose hearing is improved, compared to no treatment or placebo. The studies Plontke 2009 and Wu 2011 were included in the sensitivity analysis and confirmed this result (RR 4.21, 95% CI 1.44 to 12.31; 76 participants; 2 studies; I2 = 0%).
3.2. Analysis.

Comparison 3: Intratympanic corticosteroids compared to no treatment/placebo as secondary therapy, Outcome 2: Proportion improved
Final hearing threshold with pure tone audiometry (pure tone average)
Five studies (203 participants) were included in the meta‐analysis. The studies Ho 2004 and Chang 2010 could not be included since they did not report a final PTA. The mean final PTA at 29 to 61 days (range) after the start of therapy was lower (better) in participants with intratympanic therapy, and the point estimate of effect exceeded the minimally important difference of ‐10 dB HL (MD ‐11.09, 95% CI ‐17.46 to ‐4.72; 203 participants; 5 studies; I2 = 0%; low‐certainty evidence) (Analysis 3.3). Secondary intratympanic therapy may result, therefore, in lower (improved) final hearing thresholds compared to no treatment or placebo. The studies Plontke 2009 and Wu 2011 were included in the sensitivity analysis and confirmed the result of the primary analysis (MD ‐10.20 dB, 95% CI ‐19.64 to ‐0.77; 76 participants; 2 studies; I2 = 0%).
3.3. Analysis.

Comparison 3: Intratympanic corticosteroids compared to no treatment/placebo as secondary therapy, Outcome 3: Final PTA
Change in hearing threshold with speech audiometry
One study reported on this outcome (Plontke 2009). The speech reception threshold may be lower (better) in the group who received intratympanic treatment compared to those who received placebo, although the confidence interval crosses unity (MD ‐12.80 dB, 95% CI ‐30.17 to 4.57; 21 participants; 1 study; Analysis 3.4).
3.4. Analysis.

Comparison 3: Intratympanic corticosteroids compared to no treatment/placebo as secondary therapy, Outcome 4: Change in hearing threshold with speech audiometry
The same study also reported on the change in maximum speech discrimination, measured as the number of monosyllables understood. Again, this outcome appeared to favour intratympanic treatment (Analysis 3.5).
3.5. Analysis.

Comparison 3: Intratympanic corticosteroids compared to no treatment/placebo as secondary therapy, Outcome 5: Speech audiometry: additional outcomes
Frequency‐specific changes with pure tone audiometry
A single study reported on frequency‐specific changes with pure tone audiometry. The results are shown in Analysis 3.6.
3.6. Analysis.

Comparison 3: Intratympanic corticosteroids compared to no treatment/placebo as secondary therapy, Outcome 6: Frequency‐specific changes with PTA
Mean level of improvement, in those whose hearing is improved
This outcome was not reported by any of the included studies.
Percentage of patients reaching serviceable hearing
This outcome was not reported by any of the included studies.
Effect on tinnitus and vertigo
This outcome was not reported by any of the included studies.
Minor and serious adverse events
For this comparison, five studies provided information about adverse events (Ho 2004; Li 2011; Plontke 2009; Wu 2011; Xenellis 2006). There were insufficient data for meta‐analysis. In all studies, reporting was incomplete for one or more adverse event outcomes, either because a rate was not provided for both randomised groups, or because it was unclear in which group (or groups) events were observed.
Persistent tympanic membrane perforation
Five studies reported a rate of tympanic membrane perforation of between 0% (0/19) and 4.2% (1/24) for those who received an intratympanic injection (Ho 2004; Li 2011; Plontke 2009; Wu 2011; Xenellis 2006). This includes participants who received placebo intratympanic injection. We concluded that the evidence is very uncertain regarding the risk of tympanic membrane perforation for those who received intratympanic injection (either corticosteroid or placebo) as secondary treatment (185 participants; 5 studies; very low‐certainty evidence).
Vertigo/dizziness at the time of intratympanic injection
Three studies reported a rate of vertigo/dizziness of between 6.7% (1/15) and 33% (number not reported) for those who received an intratympanic injection (Ho 2004; Li 2011; Wu 2011). This includes participants who received placebo intratympanic injection. We concluded that the evidence is very uncertain regarding the risk of vertigo/dizziness at the time of intratympanic injection (either corticosteroid or placebo) as secondary treatment (118 participants; 3 studies; very low‐certainty evidence).
Ear pain at the time of intratympanic injection
One study reported no participants with ear pain at the time of intratympanic injection (0/24) (Li 2011). The evidence is very uncertain regarding the risk of ear pain at the time of intratympanic corticosteroid injection as secondary treatment (44 participants; 1 study; very low‐certainty evidence).
Table 6 provides details of the more limited data on other reported adverse events.
Intratympanic corticosteroids versus systemic corticosteroids as secondary therapy
No study compared the effects of intratympanic corticosteroids versus systemic corticosteroids on hearing improvement for secondary therapy of ISSNHL.
Intratympanic plus systemic corticosteroids (combined therapy) versus no treatment or versus placebo as secondary therapy
No study compared the effects of intratympanic corticosteroids plus systemic corticosteroids (combined therapy) versus no treatment or versus placebo on hearing improvement for secondary therapy of ISSNHL.
Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as secondary therapy
One study compared the effects of a secondary systemic versus a secondary combined intratympanic and systemic corticosteroid treatment (Zhou 2011).
Change in hearing threshold with pure tone audiometry (pure tone average)
Change in hearing threshold (PTA change) was not reported in the study Zhou 2011.
Proportion of patients whose hearing is improved
One study (76 participants) explored this outcome. A higher proportion of participants with combined therapy had improved hearing at 56 days after the start of treatment compared with the systemic treatment group. The point estimate of effect exceeded the minimally important difference of 25% (RR 2.24, 95% CI 1.10 to 4.55; 76 participants; 1 study; very low‐certainty evidence) (Analysis 4.1). Secondary combined therapy may therefore increase the proportion of patients whose hearing is improved compared to systemic corticosteroids alone, but the evidence is very uncertain.
4.1. Analysis.

Comparison 4: Combined compared to systemic corticosteroids as secondary treatment, Outcome 1: Proportion improved
Final hearing threshold with pure tone audiometry (pure tone average)
This outcome was not reported by Zhou 2011.
Change in hearing threshold with speech audiometry
Zhou 2011 did not assess hearing thresholds, but did report the proportion of participants who achieved an improvement of at least 15% in their speech discrimination score (see Analysis 4.2).
4.2. Analysis.

Comparison 4: Combined compared to systemic corticosteroids as secondary treatment, Outcome 2: Speech audiometry: additional outcomes
Change in speech discrimination scores
This outcome was not reported by Zhou 2011.
Frequency‐specific changes with pure tone audiometry
This outcome was not reported by Zhou 2011.
Mean level of improvement, in those whose hearing is improved
This outcome was not reported by Zhou 2011.
Percentage of patients reaching serviceable hearing
This outcome was not reported by Zhou 2011.
Effect on tinnitus and vertigo
This outcome was not reported by Zhou 2011.
Minor and serious adverse events
Zhou 2011 provided data for this comparison.
Persistent tympanic membrane perforation
The rate of tympanic membrane perforation in the intervention group was 8.1% (3/37). We concluded that the risk of tympanic membrane perforation among those who receive intratympanic corticosteroid combined with systemic corticosteroid as primary treatment is very uncertain (76 participants; 1 study; very low‐certainty evidence).
Table 8 provides details of the more limited data on other reported adverse events.
Discussion
Summary of main results
We identified data for four of our proposed comparisons, from a total of 30 randomised controlled trials (RCTs) that analysed 2133 participants. No data were found for the comparisons of intratympanic corticosteroids versus no treatment/placebo as primary therapy, intratympanic corticosteroids versus systemic corticosteroids as secondary therapy, or intratympanic corticosteroids plus systemic corticosteroids versus placebo/no treatment as either primary or secondary therapy.
The following is a summary of the key findings for each comparison:
Intratympanic corticosteroids versus systemic corticosteroids as primary therapy
We identified 16 studies and analysed 1108 patients for this comparison (Table 1). Intratympanic corticosteroids may result in a trivial or no difference in the change in hearing threshold, as compared with systemic steroids (low‐certainty evidence). They probably also result in little to no difference in the number of participants whose hearing improves, and may result in little to no difference in the final hearing threshold. The confidence intervals of all outcomes do not overlap the thresholds for clinical relevance, and these results persisted after sensitivity analysis. Overall, vertigo and dizziness may be increased, and ear pain is probably more common for those who receive intratympanic corticosteroids. However, adverse effects commonly associated with steroid use (such as blood glucose problems) may be reduced among those who receive intratympanic steroids. Persistent tympanic membrane perforation, ear pain at the time of the injection and vertigo/dizziness at the time of the injection were noted among those who received intratympanic injection, but we could not be certain how often these effects would occur.
Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as primary therapy
We identified 10 studies and analysed 788 patients for this comparison (Table 3). The change in hearing threshold may be slightly increased (better) among those who received combined therapy, but it is unclear whether this increase would be noticeable and important to patients (low‐certainty evidence). The evidence regarding the number of patients whose hearing improved, and the final hearing threshold, was very uncertain, although both outcomes favoured the combined treatment group. Adverse effects were only reported for those who received combined therapy, therefore we were unable to compare the intervention to systemic corticosteroids. Persistent tympanic membrane perforation, vertigo/dizziness at the time of the injection and ear pain at the time of the injection were all reported in the intervention group, but we could not be certain how often these effects would be seen.
Intratympanic corticosteroids versus no treatment or versus placebo as secondary therapy
Seven studies were included for this comparison (Table 5). Five studies compared the efficacy of a secondary intratympanic corticosteroid with no therapy and two studies compared intratympanic corticosteroid with intratympanic placebo. Intratympanic therapy may result in a small improvement in the change in hearing threshold (low‐certainty evidence) although the mean difference was just below the threshold for clinical relevance (PTA change ‐9.07 dB). In addition, intratympanic corticosteroids may result in a much higher proportion of patients achieving an improvement in their hearing (absolute effect of 315 more patients per 1000 having improved hearing) and a small, but clinically important, effect on the final hearing threshold (decrease of 11.09 dB HL, low‐certainty). The clinically relevant effects persisted after sensitivity analysis. Adverse effects were only reported for those who received intratympanic corticosteroids, therefore we were unable to compare the intervention to placebo or no treatment. Persistent tympanic membrane perforation, vertigo/dizziness at the time of the injection and ear pain at the time of the injection were all reported in the intervention group, but we could not be certain how often these effects would be seen. The results indicate that there may be a small improvement in hearing with the use of intratympanic corticosteroids, but it is unclear whether this would be a noticeable or important difference.
Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as secondary therapy
We identified one study and analysed 76 patients for this comparison (Table 7). Combined therapy may increase the proportion of patients whose hearing is improved, but the evidence is very uncertain. No data were available for the remaining efficacy outcomes for this comparison (change in hearing threshold or final hearing threshold, determined by PTA). The study did report that a number of participants had a persistent tympanic membrane perforation, but the evidence was very uncertain.
Overall completeness and applicability of evidence
The available evidence included all corticosteroids known to be used for intratympanic applications. All studies only included participants with sudden idiopathic sensorineural hearing loss (without alternative diagnoses) and most studies included adults. As participants included in the studies were predominantly adults, it is not clear whether these results also apply to children. However, ISSNHL in children is rare. The available evidence included patients mostly treated in secondary and tertiary care settings. In summary, we conclude that the ISSNHL patients included in the review cover the patient population seen in clinical practice.
The included studies showed variability in their treatment protocols. The glucocorticoids used in the included RCTs were either methylprednisolone or dexamethasone preparations. The methylprednisolone concentrations used were usually 40 mg/mL with only very few studies using higher concentrations (62.5 mg/mL or 125 mg/mL). The dexamethasone concentrations used were usually 4 mg/mL to 5 mg/mL with only one study using a higher concentration (12 mg/mL). We are aware that in clinical practice, other (higher) concentrations of corticosteroids might be used or recommended (Chandrasekhar 2019). In addition, other types of corticosteroids (e.g. triamcinolone acetonide), other forms of corticosteroids (e.g. dexamethasone phosphate versus dexamethasone base) and other drug delivery systems different from intratympanic injections of solutions, including wicks, gels, catheters or biodegradable controlled‐release implants, may be used (reviewed in: El Kechai 2015; Mäder 2018; Salt 2009; Salt 2018; Zhang 2021). However, we did not find any further RCTs that have addressed therapeutic strategies using intratympanic application of corticosteroids for ISSNHL, other than those included in this systematic review.
Three outcome parameters for the evaluation of the treatment effect could be used for statistical analysis (the primary outcome parameter and two secondary outcome parameters). The primary outcome parameter (change in pure tone average (PTA)) is widely used in studies on the treatment of ISSNHL. The proportion of patients whose hearing is improved (one of the secondary outcome parameters) is also a widely used outcome parameter in studies. However, in the view of the authors, this type of outcome parameter is not very reliable because 1) it is a dichotomous parameter, which gives only a little information about the absolute hearing improvement in patients and within‐study group variance and 2) it is highly dependent on the definition of hearing improvement, which is inconsistent between studies. As a further outcome parameter we included the final PTA in patients at the study endpoint. Although this outcome parameter is not widely used in studies, it has been shown to depend less upon baseline characteristics (such as initial hearing loss or treatment delay), and is therefore more robust against distortions due to differences in baseline characteristics between treatment arms (Liebau 2017; Liebau 2018).
In this review, the wording of the comments in the summary of findings tables and, thus, in the abstract, results and authors’ conclusions sections is based on the "GRADE guidelines informative statements to communicate the findings of systematic reviews of interventions" (Santesso 2020). In this guideline, producers and users of systematic reviews found statements to communicate findings that combine the size and certainty of an effect to be acceptable. The final list of informative statements to communicate the results of systematic reviews combines the effect size (1) large effect, 2) moderate effect, 3) small important effect, 4) trivial, small unimportant effect or no effect) and the certainty of the evidence (high, moderate, low, very low) (Santesso 2020). The Cochrane Handbook for Systematic Reviews of Interventions (Chapter 15.6.4) also suggests using these narrative statements to draw conclusions based on the effect estimate from the meta‐analysis and the certainty of the evidence (Handbook 2021). The clinicians amongst the authors initially tried to define the effect sizes in detail (1) large effect, 2) moderate effect, 3) small important effect, 4) trivial, small, unimportant effect or no effect) for both change in hearing threshold and proportion of patients whose hearing improved. However, we could not agree on a uniform statement for this. Thus, we agreed on a 'minimally important difference' (MID).
Determining a relevant and important change in hearing is challenging. In this review we have taken a change in hearing threshold of 10 dB HL to represent the MID. However, we acknowledge that this may not be universally agreed. The decision to choose 10 dB as a MID was based on the test‐retest reliability of pure tone audiometric measurements, established minimal criteria for improvement in individual patients (Chandrasekhar 2019; Gurgel 2012; Stachler 2012), and on a large RCT on this topic with low bias (Rauch 2011). For dichotomous outcomes (e.g. the proportion of patients with hearing improvement), we used a threshold of 25% or more in RR increase for appreciable benefit as suggested in the GRADE guideline (Guyatt 2011). The 10 dB difference and the 25% criteria were agreed upon by all authors. Many of the mean differences reported in this review were close to this MID, therefore it is uncertain whether the detectable change from the interventions would be of importance to patients.
Some studies used different thresholds to define 'improvement' of hearing. This may result in different conclusions to this review. If, for example, a change of 5 dB HL (or 3 dB, 6 dB or 9 dB) was deemed to be the MID then we would have concluded that some interventions were of more certain benefit. This may also partly explain apparent discrepancies in our findings where the mean difference was found not to be clinically relevant, and yet a higher proportion of patients 'improved' when assigned to the intervention group. When interpreting the findings, it is important to consider both the mean change in hearing and how many people improved. Although the mean change for the whole group may not be especially strong, there may still be a greater number of people who improve.
The estimation of whether an effect size is "1. large", "2. moderate", "3. small important" or "4. trivial, small unimportant effect or no effect" (Santesso 2020) also depends on the degree of initial hearing loss (i.e. moderate, severe, profound hearing loss) and whether the patients had serviceable hearing before and/or after therapy (Chandrasekhar 2019). For example, a 10 dB change might not be useful in severe or profound hearing loss if the patient (or the ear) would remain at a cochlear implant candidate level after therapy. The current US guideline therefore correctly recommends that future studies should report the number of patients reaching serviceable hearing: "For ears that were rendered nonserviceable by the episode of SSNHL, return to serviceable hearing should be considered a significant improvement, and whether or not this level of recovery occurs should be recorded. Recovery to a serviceable level typically indicates that after recovery, the ear would be a candidate for traditional hearing amplification. Recovery to less‐than‐serviceable levels indicates an ear that would, in most circumstances, not benefit from traditional amplification. For ears with SSNHL to hearing levels that are still in the serviceable range, an improvement of > 10 dB in pure tone thresholds (accounting for test‐retest variability in audiometry) or an improvement in WRS of > 10% (approximate lower limit for a statistically significant change based on binomial tables for WRS of >50% at baseline) should be considered partial recovery and recorded." (Chandrasekhar 2019).
We think that the criteria with high patient relevance are: how many patients (ears) reach levels where they are not a cochlear implant candidate anymore ("serviceable hearing", as stated above) or even reach levels where a hearing aid would not be necessary any more? Such criteria mainly depend on word recognition tests, which were not sufficiently reported in the RCTs in our review. However, speech audiometry results are difficult to compare due to different test strategies and different languages. The criteria for candidacy for a cochlear implant or a hearing aid may also differ between countries or even between audiologists.
Data for many of the outcomes were missing. The length of follow‐up in studies was less than a year, meaning that there was limited evidence regarding the long‐term effectiveness of the therapies. However, a stable hearing threshold is considered to occur several weeks after treatment of ISSNHL and long‐term follow‐up may increase the likelihood of occurrence of other causes of hearing loss, which would confound any long‐term analysis.
Important or key outcome criteria missing in this review are quality of life and patient‐reported outcome measures. These measures should ideally have been defined in the protocol version of this review. Possible tools for measuring quality of life or patient‐reported outcomes might be the Hearing Handicap Inventory for the Elderly (Ventry 1982), the Short Form (12) Health Survey (SF‐12) (Jenkinson 1997), or the Core Rehabilitation Outcome Set for Single Sided Deafness (CROSSSD) (Katiri 2020). However, quality of life measures had not been reported in any of the RCTs included in this systematic review.
Quality of the evidence
We largely assessed the certainty of the evidence in this review as low or very low. There was moderate‐certainty evidence for a small number of outcomes, but we identified no high‐certainty evidence. The main reasons for the uncertainty were a serious risk of bias in the included studies and imprecision in the effect estimates ‐ either due to a small number of included participants or few events, or because the wide confidence intervals overlapped the threshold for clinical relevance.
Nearly all the included studies had a small number of participants. This increases the risk that randomisation does not achieve balance across groups for important prognostic characteristics (both known and unknown) that may confound outcome estimates. One such characteristic is the propensity towards spontaneous recovery. This can have a large impact on pooled estimates for hearing threshold, and imbalance across groups may not be detected by a comparison of baseline parameters such as pre‐treatment hearing loss and delay between the onset of symptoms and the start of treatment. It is important, therefore, to make sure that trials have enough participants to achieve a balance across groups for the propensity towards spontaneous recovery, as well as other important prognostic factors. This is supported by meta‐analyses (Liebau 2017; Liebau 2018), which demonstrated (in primary and secondary treatment respectively) that variation in outcome estimates is reduced when there is a larger number of participants.
These observations are reflected in the meta‐analysis as well. In many included studies a high within‐group variance was seen that might be the consequence of the heterogeneous impact of spontaneous recovery on total hearing improvement among patients, in combination with a low number of included participants per treatment arm. In addition, heterogeneous results were found in outcome parameters between studies, especially in studies with a small number of included participants. However, due to the high imprecision of the results in these studies statistical heterogeneity might be underestimated by the Chi² test and I² statistic.
We created funnel plots for outcomes including 10 or more studies (Figure 4; Figure 5; Figure 6). These did not indicate the presence of publication bias. However, this is not proof that no publication bias exists. It is noteworthy that we found some high‐quality studies with larger sample sizes indicating no differences between treatment modalities and smaller studies with higher risk of bias indicating large differences in treatment effects. High‐quality studies with large sample sizes imply more investment of time, work and money. It is very likely that these studies will be published afterwards. In smaller studies, a higher risk exists that the results of those studies will not be published if the conclusion differs from current concepts or expectations.
4.

5.

6.

The overall methodological and reporting quality of the studies was disappointing. This leads to an overall high risk of bias in most of the included studies and reduces the certainty of the evidence. It seems that 20 years after CONSORT (Begg 1996), authors and journals in the field of otolaryngology still do not adhere to these guidelines and most of the publications of RCTs were accepted without fulfilling essential methodological and reporting criteria. Further, only very few included studies were reported to be pre‐registered. Without publishing a pre‐specified study protocol, uncertainty remains about whether study methods may have changed after data synthesis and before publishing the study results.
Many studies did not report their method of randomisation. It was therefore not possible to justify whether an adequate randomisation method was used in these studies. Similarly, almost no study reported their method for concealment of allocation. However, the randomisation principle is only guaranteed if ‐ in addition to a robust method of randomisation ‐ a plausible method was used that prevents manipulation of the allocation process. Without this information the randomisation procedure remains questionable. In addition, some studies reported an inadequate method of randomisation in which the allocation of patients could be predicted (such as alternate allocation).
It is also mandatory to give a clear statement about which persons in the study are blinded and which are not. Blinding of outcome assessment is possible even in non‐placebo‐controlled studies. Without a clear statement of blinding a judgement on the risk of bias is not possible and remains unclear.
In some of the included studies, treatment arms actually differed considerably in their baseline parameters and these imbalances between groups may have influenced the reported outcomes. In addition, many studies have not reported important baseline parameters, or reported them in an inadequate way. In two studies the reporting of baseline parameters even differed between treatment arms. Such inconsistent reporting of data raises doubts about the transparency of studies. We also noted a number of studies where measures of variance (such as standard deviations or standard errors) were missing. A variance element is an essential component when reporting the mean.
Potential biases in the review process
The included studies used a broad range of different treatment protocols and follow‐up times for final hearing evaluation. No specific conclusions can thus be drawn on the effectiveness of a particular corticosteroid treatment protocol for ISSNHL. However, this only marginally influences the general conclusions on the comparison of treatment modalities (i.e. systemic, intratympanic or combined treatment).
The included studies also cover a broad range of baseline characteristics (e.g. degree of hearing loss, treatment delay, type of primary treatment for comparisons using intratympanic corticosteroids as secondary treatments, and accompanying symptoms such as vertigo).
There are differences between studies concerning hearing evaluation (different frequencies used for calculating PTA) and different definitions of positive response to treatment (proportion of patients whose hearing is improved). It has previously been shown that the frequencies chosen for calculating the PTA influence the estimation of hearing loss in patients and thus can influence the outcome in clinical trials (Plontke 2007). Different definitions of positive response to treatment can present a risk of bias when pooling the outcome parameter 'proportion of patients improved' (Haynes 2007).
There are many included studies with a high risk of bias and major methodological weaknesses. We therefore performed a sensitivity analysis, which takes into account only studies with high quality and low risk of bias.
One included study was performed by authors of the review (SKP and CM) (Plontke 2009). These authors were therefore not involved in data extraction or risk of bias assessment for this study.
The outcome parameter 'final PTA' after treatment was added after the publication of the protocol. This was based on conclusions drawn from a different meta‐analysis (Liebau 2017; Liebau 2018), and was independent from the data processing in the present meta‐analysis.
Some of the planned secondary outcomes could not be assessed due to the lack of available data. Due to missing individual patient data in almost all studies and the limited number of studies per type of comparison, intended subgroup analyses could not be performed either. However, this has no influence on the conclusions drawn from the overall analysis.
Agreements and disagreements with other studies or reviews
A number of meta‐analyses on local corticosteroid treatment of ISSNHL have already been published. There is much variation concerning included studies, defined types of comparisons and evaluated outcome parameters within these reviews. However, the primary outcome parameter was either mean hearing gain (change in hearing threshold with pure tone audiometry) or recovery rate (proportion of patients whose hearing is improved). Many studies included both outcome parameters.
Intratympanic corticosteroids versus no treatment or versus placebo as primary therapy
We identified no data of relevance for this comparison because of the lack of studies that fulfilled the inclusion criteria. Despite that, the meta‐analysis Ahmadzai 2019 reports a significant benefit of intratympanic treatment over placebo in hearing gain and recovery rate. They did not perform pair‐wise comparisons in their meta‐analysis but pooled single treatment arms from different trials instead. Besides inclusion of the study Filipo 2013 with an early salvage therapy at day seven, they further included the placebo groups from the studies Hultcrantz 2014 and Nosrati‐Zarenoe 2012, although these studies compared the efficacy of systemic corticosteroids versus placebo instead of local treatment. This may lead to a greater risk of bias because effect evaluation is not based on a randomised process.
Intratympanic corticosteroids versus systemic corticosteroids as primary therapy
A large number of meta‐analyses have examined the difference in effectiveness of intratympanic and systemic treatment as primary therapy. Most of them are in accordance with our finding of no important difference between the two treatment modalities (Ahmadzai 2019; Crane 2015; El Sabbagh 2016; Garavello 2012; Lai 2017; Mirian 2020). However, the meta‐analysis Crane 2015 included studies by Ahn 2008 and Arslan 2011, which compared combined rather than local treatment. The review Garavello 2012 included the study Ahn 2008 in this comparison. The meta‐analysis El Sabbagh 2016 did not separate conditions of primary and secondary therapy and pooled studies of both types of treatment. They also included studies with combined treatment in the intervention group in this type of comparison. The meta‐analyses Mirian 2020 and Lai 2017 did not include some of the trials included in our analysis. The meta‐analysis Ahmadzai 2019 pooled single treatment arms from different studies and therefore included the systemic treatment groups of Eftekharian 2016, Gundogan 2013 and Hultcrantz 2014, as well as the local treatment group from Filipo 2013 with early salvage therapy in this comparison.
The meta‐analysis Quiang 2016 found a significantly greater benefit of intratympanic therapy over systemic therapy as a primary intervention when assessed with mean hearing gain as well as recovery rate. This meta‐analysis, however, did not include the large clinical trial with low bias Rauch 2011, in which there were a high number of included participants finding no significant difference in outcomes between treatment modalities. The meta‐analysis Zhao 2016 found a significant difference in the rate of complete hearing recovery, but not in the general recovery rate, termed as significant hearing improvement. However, this review included the studies Mao 2005, Yi 2011, You 2008 and Zhou 2006. The studies Yi 2011 and Mao 2005 investigated the injection of corticosteroids through the Eustachian tube. You 2008 is a non‐randomised study, while Zhou 2006 included hyperbaric oxygen therapy in treatment arms. The meta‐analysis Li 2020 reported a significant difference in mean hearing gain but not in recovery rate. However, they did not include a number of trials that were included in our analysis.
Intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone as primary therapy
Other meta‐analyses about the effectiveness of combined therapy over systemic corticosteroids as primary therapy found heterogeneous results. This is consistent with our conclusion that there is uncertainty in the evidence. Ahmadzai 2019 and Mirian 2020 concluded that combined therapy is not superior to systemic therapy as primary treatment. On the other hand, the meta‐analyses Gao 2016, Han 2017 and Li 2020 showed a significant difference in the effectiveness of combined and systemic therapy (favouring combined therapy) and concluded that combined therapy might be superior to systemic therapy. Gao 2016, however, included two non‐randomised trials (Battaglia 2014; Günel 2015). In Günel 2015, the control group was a retrospective cohort. Han 2017 also included the study Ashtiani 2012, a study with a very uncertain randomisation process, which is only mentioned in the abstract, not in the full text of their publication. They also included the non‐randomised trial Battaglia 2014 and the trial Chen 2015, in which the control group included a mixture of patients receiving combined or systemic treatment. Li 2020 also included the non‐randomised trials Battaglia 2014 and Ashtiani 2012 (very uncertain randomisation process). Further, they included the trial Zhou 2011, which investigated the efficacy of combined therapy against systemic treatment as a secondary intervention.
Intratympanic corticosteroids versus no treatment or versus placebo as secondary therapy
In accordance with our meta‐analysis, other reviews also found that intratympanic salvage therapy might be more effective than no therapy or placebo therapy. The meta‐analyses Ng 2015 and Li 2015 found a significantly higher mean change in hearing threshold in patients receiving intratympanic therapy. The meta‐analysis Spear 2011 also found a significant difference in mean change in hearing threshold. However, this review also included non‐randomised studies (Kiliç 2007; Plaza 2007; She 2010). The reviews Crane 2015 and Garavello 2012 found a significantly higher recovery rate in patients receiving intratympanic therapy. The review Crane 2015, however, included the study Zhou 2011, which compared combined treatment with systemic therapy. The review Garavello 2012 also included the study Zhou 2011 and the study Arslan 2011, which compared combined therapy with systemic treatment as primary therapy.
Combined intratympanic plus systemic corticosteroids versus systemic corticosteroids alone as secondary therapy
We found no review addressing this question.
Authors' conclusions
Implications for practice.
For primary therapy, we identified no evidence on the efficacy of intratympanic therapy compared to placebo/no treatment.
Intratympanic corticosteroids probably result in little to no difference when compared to systemic corticosteroids in primary therapy for idiopathic sudden sensorineural hearing loss (ISSNHL). The evidence regarding adverse events was very uncertain.
The evidence regarding combined corticosteroid therapy was very uncertain. There may be a slight benefit to combined therapy when compared to systemic corticosteroids alone, but the difference may be small. For change in hearing threshold and for final hearing threshold, the mean difference between the two groups is close to the threshold for a minimally important difference (estimates of ‐8.55 dB and ‐9.11 dB, respectively) and it is unclear whether it would be important to patients. The evidence regarding adverse events was very uncertain.
For secondary therapy, intratympanic corticosteroid therapy may be more effective than no treatment or placebo for ISSNHL. Intratympanic therapy may result in a much higher proportion of participants whose hearing is improved and for final hearing threshold the difference exceeds the threshold for a minimally important difference (estimates of ‐11.09 dB HL). For change in hearing threshold, the mean difference between the two groups is close to the threshold for a minimally important difference (estimates of ‐9.07 dB).
The evidence regarding adverse events was very uncertain.
We are very uncertain about the effect of combined therapy on hearing outcome for secondary therapy of ISSNHL when compared to systemic therapy. The change in hearing threshold may be slightly increased, but it is not clear whether the extent of change would be important to patients. The evidence regarding adverse events was very uncertain.
Further research is likely to have an important impact on the estimates of effect and may change the estimates in the respective comparisons.
Implications for research.
Suggestions for future trials
Design and methods
Where the intent is to assess the effectiveness of interventions, randomised controlled trials should be conducted. Trials should use appropriate methods for randomisation and allocation concealment to avoid selection bias, and they should be adequately powered. Attempts should be made by the investigators to blind participants, healthcare professionals and study personnel to the treatment allocation. This could be through the use of a placebo and ensuring that the treatment regimens are the same between treatment arms. A double placebo design should be used where dosage form and/or regimen are different. Where it is not possible to blind participants and/or clinicians to the treatment received, efforts to blind the outcome assessment and analysis personnel should be made.
Populations
Populations should be clearly described with respect to the degree of initial hearing loss and additional symptoms. A standardised and evidence‐based definition for ISSNHL, especially with respect to audiological criteria, still needs to be established.
Interventions
There should be clear reporting of the therapies used, including the drug, dose, frequency and duration, and clear descriptions of any adjunctive therapies used across the treatment groups. Publications should make it clear exactly which form of the drug (the exact chemical composition) was used in the study. This should apply to any drug used, not just corticosteroids (Salt 2020). We recommend gathering evidence on the various corticosteroid treatment regimens including various concentrations/dosages, forms of corticosteroids, injection frequencies and intervals, drug formulations and delivery systems, and (other) methods for enhancing uptake into the cochlea.
Outcomes
Primary and secondary outcomes should be clearly defined and these parameters should also be evaluated later on. The development of core outcome sets for ISSNHL would be beneficial for future trials. This would help to ensure that trials are consistent, high‐quality and examine appropriate outcomes. Fixed levels of improvement may not always be adequate, since benefit for patients depends on the initial degree of hearing loss and the final outcome, respectively. Speech audiometry in quiet and noise are preferable over pure tone thresholds. Internationally comparable speech audiometry tests should be further developed and applied if possible (Akeroyd 2015; Kollmeier 2015). Other categorical criteria of high patient relevance, such as the necessity of an ear still being a cochlear implant or hearing aid candidate after partial recovery, should be considered (see Discussion and Chandrasekhar 2019). Consensus should also be reached on the appropriate minimally importance difference (appreciable or clinically relevant benefit and harm) to be used when assessing hearing outcomes.
The primary endpoint should not be too early for ISSNHL studies. We suggest a primary outcome assessment between four weeks and three months, and studies should follow up patients for at least six months. Efforts should be made to establish internationally comparable speech audiometry tests including speech understanding in noise.
The assessment of adverse effects should be defined in the protocol and these should be systematically sought during trials. A validated patient‐reported outcome measure (PROM) or quality of life measurement instrument should be used whenever possible.
Reporting
Trials should be registered in a regional or international clinical trials registry and, when published, adhere to reporting guidelines such as CONSORT (Schulz 2010).
History
Protocol first published: Issue 4, 2009
Acknowledgements
This project was supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF), grant 01KG2019 (ITKORT) to Stefan K Plontke, and by the State of Saxony‐Anhalt, Germany, through research funding of the Martin Luther University Halle‐Wittenberg (SKP, AL and JR).
This project was also supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We wish to thank Jenny Bellorini and Katie Webster for their continuous editorial help and Samantha Cox and Gemma Sandberg for their help with creating the search strategies and for help with the searches.
We are grateful to Roland Zeh as a representative of the following patient organisations providing advice on quality of life (QoL) and patient‐relevant outcome criteria (PROM): German Cochlear Implant Society (Deutsche Cochlear Implant Gesellschaft e.V., DCIG, Senden); German Alliance of Hearing Impaired (Deutscher Schwerhörigenbund e.V, DSB, Berlin); German Society of Hearing Impaired (Deutsche Gesellschaft der Hörgeschädigten ‐ Selbsthilfe und Fachverbände e.V., DG, Rendsburg).
We are grateful to Yuan Chi, Centre for Evidence‐Based Chinese Medicine, Beijing University of Chinese Medicine, Zhengjie Li and Zhaoli Meng for translating and identifying primary studies for inclusion or exclusion for this review.
We are grateful to Yuan Chi, Centre for Evidence‐Based Chinese Medicine, Beijing University of Chinese Medicine and Zhaoli Meng for data extractions for Chang 2020, Diao 2012, Peng 2008 and Qu 2015.
We are grateful to You‐Shan Feng, Institute for Clinical Epidemiology and Applied Biometry, University Hospital Tübingen, for identifying primary studies for inclusion or exclusion for this review.
Editorial and peer reviewer contributions
Cochrane ENT supported the authors in the development of this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Martin Burton, Cochrane ENT & Cochrane UK
Managing Editor (selected peer reviewers, collated peer reviewer comments, provided editorial guidance to authors, edited the article): Jenny Bellorini, Cochrane ENT
Copy Editor (copy editing and production): Jenny Bellorini, Cochrane ENT
Peer reviewers (provided comments and recommended an editorial decision): Richard Rosenfeld (Cochrane ENT Editor), Adrian James (Cochrane ENT Editor), Sujana S Chandrasekhar, MD Partner, ENT & Allergy Associates, LLP Clinical Professor of Otolaryngology‐HNS, Zucker School of Medicine at Hofstra‐Northwell (external clinical/content review).
Appendices
Appendix 1. Search strategies
| CENTRAL | PubMed | Embase (Ovid) |
| 1 MESH DESCRIPTOR Hearing Loss, Sudden EXPLODE ALL AND CENTRAL:TARGET 2 MESH DESCRIPTOR Hearing Loss, Sensorineural EXPLODE ALL AND CENTRAL:TARGET 3 (sudden*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 4 #2 AND #3 AND CENTRAL:TARGET 5 (sshl OR snhl OR ishl OR isshl OR issnhl OR ssnhl):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 6 (sudden* AND (hearing OR deaf)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 7 #4 OR #1 OR #5 OR #6 AND CENTRAL:TARGET 8 MESH DESCRIPTOR Administration, Topical EXPLODE ALL AND CENTRAL:TARGET 9 MESH DESCRIPTOR Injection, Intratympanic EXPLODE ALL AND CENTRAL:TARGET 10 (intratympanic* OR topical* OR local*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 11 #8 OR #9 OR #10 AND CENTRAL:TARGET 12 #7 AND #11 AND CENTRAL:TARGET |
#1 "HEARING LOSS, SUDDEN"[Mesh]
#2 "HEARING LOSS, SENSORINEURAL"[Mesh]) AND (sudden*))
#3 (sshl[tiab] OR snhl[tiab] OR ishl[tiab] OR isshl[tiab] OR issnhl[tiab] OR ssnhl[tiab])
#4 (sudden*[tiab] AND (hearing[tiab] OR deaf*[tiab])))
#5 #1 OR #2 OR #3 OR #4
#6 ((intratympanic*[tiab] OR topical*[tiab] OR local*[tiab])
#7 "Administration, Topical"[MeSH] #8 "Injection, Intratympanic"[Mesh] #9 #6 OR #7 OR #8 #10 #5 AND #9 |
1 sudden deafness/ 2 exp perception deafness/ 3 sudden*.tw. 4 2 and 3 5 (sshl or snhl or ishl or isshl or issnhl or ssnhl).tw. 6 (sudden* and (hearing or deaf*)).tw. 7 1 or 4 or 5 or 6 8 (intratympanic* or topical* or local*).tw. 9 topical drug administration/ 10 exp intratympanic drug administration/ 11 8 or 9 or 10 12 7 and 11 |
| Web of Science (Web of Knowledge) | ICTRP and ClinicalTrials.gov | ICTRP and ClinicalTrials.gov (CRS) |
| #1 TS=(sshl or snhl or ishl or isshl or issnhl or ssnhl) #2 TS=(sudden* and (hearing or deaf*)) #3 #1 OR #2 #4 TS= (intratympanic* or topical* or local*) #5 #3 AND #4 |
ICTRP sshl OR snhl OR ishl OR isshl OR issnhl OR ssnhl OR (sudden AND (deaf* OR hear*)) Clinicaltrials.gov (sudden AND (deafness OR hearing)) AND (local OR intratympanic OR topical) |
1 (sudden* AND (deaf* OR hearing)) AND (local* OR intratympanic* OR topical*) AND CENTRAL:TARGET 2 http*:SO AND CENTRAL:TARGET 3 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET 4 #2 OR #3 5 #1 AND #4 |
Data and analyses
Comparison 1. Intratympanic compared to systemic corticosteroids as primary therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mean change in pure tone average (PTA) | 10 | 701 | Mean Difference (IV, Fixed, 95% CI) | ‐5.93 [‐7.61, ‐4.26] |
| 1.2 Proportion improved | 14 | 972 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.97, 1.12] |
| 1.3 Final PTA | 7 | 516 | Mean Difference (IV, Fixed, 95% CI) | ‐3.31 [‐6.16, ‐0.47] |
| 1.4 Change in hearing threshold with speech audiometry | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐8.85 [‐19.58, 1.88] |
| 1.5 Speech audiometry: additional outcomes | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Speech discrimination score: change from baseline | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 15.64 [1.57, 29.71] |
| 1.5.2 Speech discrimination score: endpoint | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [‐20.88, 32.88] |
| 1.5.3 Word recognition score: change from baseline | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐9.28, 8.08] |
| 1.6 Frequency‐specific changes in PTA | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.1 0.25 kHz | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.2 0.5 kHz | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.3 1 kHz | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.4 2 kHz | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.5 3 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.6 4 kHz | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.7 8 kHz | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.8 Frequency range: low | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.9 Frequency range: mid | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.10 Frequency range: high | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.7 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Ear pain | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.68 [6.22, 39.49] |
| 1.7.2 Otitis media | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.28 [0.70, 15.49] |
| 1.7.3 Vertigo/dizziness | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.41, 4.54] |
| 1.7.4 Blood glucose problems | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.35, 0.85] |
| 1.7.5 Mood change | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.13, 0.37] |
| 1.7.6 Sleep change | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.10, 0.36] |
| 1.7.7 Appetite change | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.09, 0.44] |
| 1.7.8 Weight change | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.13, 0.61] |
| 1.7.9 Dry mouth | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.06, 0.35] |
| 1.7.10 Any adverse event | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.94, 1.12] |
| 1.7.11 Serious adverse event | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.35, 3.59] |
Comparison 2. Combined compared to systemic corticosteroids as primary therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mean change in pure tone average (PTA) | 6 | 435 | Mean Difference (IV, Fixed, 95% CI) | ‐8.55 [‐12.48, ‐4.61] |
| 2.2 Proportion improved | 10 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.15, 1.41] |
| 2.3 Final PTA | 3 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐9.11 [‐16.56, ‐1.67] |
| 2.4 Change in hearing threshold with speech audiometry | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐7.59 [‐20.22, 5.04] |
| 2.5 Speech audiometry: additional outcomes | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5.1 Speech discrimination score: change from baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5.2 Speech discrimination score: endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6 Frequency‐specific changes with PTA | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6.1 0.25 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6.2 0.5 kHz | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6.3 1 kHz | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6.4 2 kHz | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6.5 3 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6.6 4 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6.7 8 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6.8 Frequency range: low | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6.9 Frequency range: mid | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6.10 Frequency range: high | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Intratympanic corticosteroids compared to no treatment/placebo as secondary therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mean change in PTA | 7 | 280 | Mean Difference (IV, Fixed, 95% CI) | ‐9.07 [‐11.47, ‐6.66] |
| 3.2 Proportion improved | 6 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.55 [2.89, 10.68] |
| 3.3 Final PTA | 5 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐11.09 [‐17.46, ‐4.72] |
| 3.4 Change in hearing threshold with speech audiometry | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐12.80 [‐30.17, 4.57] |
| 3.5 Speech audiometry: additional outcomes | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 19.90 [0.41, 39.39] |
| 3.5.1 Maximum speech discrimination: change from baseline | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 19.90 [0.41, 39.39] |
| 3.6 Frequency‐specific changes with PTA | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6.1 0.25 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6.2 0.5 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6.3 1 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6.4 2 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6.5 3 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6.6 4 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6.7 6 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6.8 8 kHz | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Combined compared to systemic corticosteroids as secondary treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Proportion improved | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.24 [1.10, 4.55] |
| 4.2 Speech audiometry: additional outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 Improvement in speech discrimination score | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [1.12, 5.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahn 2008.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group, randomised controlled trial with 14 days duration of treatment and a 3‐month duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Republic of Korea, February 2005 to March 2007 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment:
Inclusion criteria: SSNHL with acute onset of HL of > 30 dB in 3 contiguous frequencies, which may have occurred instantaneously or progressively over several days Exclusion criteria: medical or central nervous system conditions, including diabetes, hypertension, connective‐vascular disease, vestibular schwannoma, true vertigo with whirling type, and other conditions that could affect hearing recovery or selection of therapeutic methods |
|
| Interventions |
General comparison: intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone Intervention group (n = 60*): "combined therapy": intratympanic injection of dexamethasone, 5 mg/mL, 0.3 to 0.4 mL, 3 injections total, 1 injection every 2 days (first, third and fourth day) + systemic steroid therapy as in comparator group Comparator group (n = 60*): "systemic": 14‐day course of oral 48 mg methylprednisolone for 9 days, followed by tapering for 5 days Use of additional interventions (common to both treatment arms): vitamins and lipo‐prostaglandin E1 * intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 90 days Used PTA: 4PTA (0.5, 1, 2, 3 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from protocol: "All patients are being allocated to one of two groups (ITD and control group) using randomizations table that is generated by random number generator (http://stattrek.com/statistics/random‐number‐generator.aspx)." Quote from protocol: "If a patients refuses to receive such treatment as allocated to him or her, the patient is going to be dropped from this study." |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial, no blinding. Quote from protocol: "If a patients refuses to receive such treatment as allocated to him or her, the patient is going to be dropped from this study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data reported but patients could refuse allocated therapy. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. |
Al‐Shehri 2015.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group, randomised controlled trial with 14 days duration of treatment and 2‐month duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Saudi Arabia, January 2011 to December 2014 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment:
* not clarified whether this is SD or SEM Inclusion criteria: age > 18, unilateral sensorineural hearing loss that developed within 72 hours and was present for 2 weeks or less. Patients' pure tone average (PTA) must have been 50 dB or higher, and the affected ear must have been at least 30 dB worse than the contralateral ear in at least 1 of the 4 PTA frequencies (i.e. 500, 1000, 2000 and 4000 Hz). Exclusion criteria: hearing has been asymmetric prior to the onset of ISSNHL. Pre‐enrollment steroid usage, previous history of hearing loss, Ménière's disease, or any chronic inflammatory or suppurative ear disease or cholesteatoma, otosclerosis, ear surgery (except ventilating tubes), hearing asymmetry prior to onset, congenital hearing loss, physical trauma or barotrauma to the ear immediately preceding hearing loss, history of genetic hearing loss with strong family history, or craniofacial or temporal bone malformations. |
|
| Interventions |
General comparison: intratympanic versus systemic corticosteroid therapy Intervention group (n = 19*): "intratympanic therapy": intratympanic methylprednisolone sodium succinate 4 x 1 mL doses of 40 mg/mL over 2 weeks, with a dose given every 3 to 4 days Comparator group (n = 20*): "systemic therapy": oral prednisone 60 mg/d tapering over 14 days Use of additional interventions (common to both treatment arms): none *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 75 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | None declared | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[Patients] were consecutively randomized" Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The researcher was not blinded to the treatment group but the audiologists were blinded to it." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Treatment delay from onset in patients not reported. Single authorship generally exhibits a certain risk of bias. Completely equal baseline PTA is notable. |
Arastou 2013.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 10 days duration of treatment and 2 weeks duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Iran, June 2008 to November 2009 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment:
Inclusion criteria: ISSNHL that developed within 24 h, without identifiable cause including retrocochlear disease or trauma. Participants were eligible for inclusion in the study if they had at least one poor prognostic factor: age greater than 40 years, hearing loss more than 70 dB, or greater than a 2‐week delay between the onset of hearing loss and initiation of therapy. Exclusion criteria: hypertension, diabetes mellitus, tympanic perforation in the affected ear, history of surgery on the affected ear, bilateral SSNHL, ISSNHL in the hearing ear only, if they were pregnant, or if they received any therapy for SSNHL prior to enrolment in the study. |
|
| Interventions |
General comparison: intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone Intervention group (n = 36*): "combined therapy": intratympanic injection of dexamethasone, 4 mg/mL, 0.4 mL, 4 injections total, twice per week + systemic steroid therapy as in comparator group Comparator group (n = 41*): "systemic therapy": prednisolone oral 1 mg/kg per day for 10 days Use of additional interventions (common to both treatment arms): acyclovir (4 x 0.5 g/d for 10 days), triamterene H, omeprazole (daily during steroid treatment) *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 14 days Used PTA: 5PTA (0.25, 0.5, 1, 2, 4 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized ... using a series of computer generated numbers" |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The audiologist was blinded to the study group of the patient." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All participants received the therapy to which they were randomized, and all patents completed the therapy". |
| Selective reporting (reporting bias) | High risk | Missing standard deviation in reporting of hearing improvement. |
| Other bias | High risk | No sample size calculation performed. Unexplained difference in numbers of patients between groups. Wide range of treatment delay from onset among included patients. |
Arslan 2011.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 10 days duration of treatment and 15 days duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Turkey, January 2003 to October 2008 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment:
Inclusion criteria: ISSNHL with minimum 20 dB hearing loss in 3 consecutive octaves that have occurred within a course of 3 days Exclusion criteria: history, symptoms or findings of acoustic trauma or barotrauma, Ménière's disease or other peripheral vertigo, tumours, autoimmune disease, coagulopathy or small vessel disease, syphilis, hypothyroidism and ototoxic drug use, contraindication to use systemic steroids |
|
| Interventions |
General comparison: primary intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone Intervention group (n = 85*): "combined therapy": intratympanic injection of methylprednisolone, 125 mg per mL, 0.5 mL, 5 times total, 1 injection every 2 days + systemic steroid therapy as in comparator group Comparator group (n = 73*): "systemic therapy": intravenous then oral methylprednisolone 100 mg intravenous first day, 80 mg per day oral in 3 divided doses for the next 2 days, continued with oral administration by tapering the dose 20 mg in every 2 days Use of additional interventions (common to both treatment arms): dextran 40,000, 5 mL/kg per day intravenous, for the first 5 days of treatment *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 15 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "randomised according to their date of referral … at odd and even days" |
| Allocation concealment (selection bias) | High risk | No allocation concealment possible because of the method of random sequence generation used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Difference in numbers of patients between groups. Early endpoint of 15 days after start of treatment only. Wide range of treatment delay from onset among included patients. Hearing threshold inclusion criteria is as low as only > 20 dB HL in 3 octaves. |
Ashtiani 2018.
| Study characteristics | ||
| Methods | Triple‐blinded, parallel‐group randomised controlled trial with 13 days duration of treatment and 4 weeks duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Iran, 2011 to 2014 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment/baseline median SRT/baseline median SDS:
*mild (26 to 40 dB HL), moderate (41 to 55 dB HL), moderately severe (56 to 70 dB HL), severe (71 to 90 dB HL), profound (≥ 91 dB HL) Inclusion criteria: SSNHL and age of at least 18 years and a unilateral sensorineural hearing loss that developed within 72 h and was present for 10 days or less Exclusion criteria: receiving oral or injection treatments at other centres and having contraindications for corticosteroid therapy (such as pregnancy or glaucoma), a previous history of SSNHL, an immunodeficiency, a history of fluctuating hearing loss, a history of endolymphatic hydrops, brain or temporal bone pathology in the MRI, and concurrent otitis (acute and chronic) and being unco‐operative or visiting with a delay of 10 days or more |
|
| Interventions |
General comparison: intratympanic versus combined versus systemic corticosteroid therapy Intervention group I (n = 32*): "intratympanic therapy": intratympanic 0.6 mL vials of methylprednisolone in the anterior superior (AS) TM on days 1, 5, 9 and 13, oral 75 mg/day placebo over 10 days Intervention group II (n = 35*): "combined therapy": intratympanic 0.6 mL vials of methylprednisolone in the anterior superior (AS) TM on days 1, 5, 9 and 13, oral 75 mg/day prednisolone over 10 days Comparator group (n = 45*): "systemic therapy": oral 75 mg/day prednisolone over 10 days, intratympanic 0.6 mL of placebo in the anterior superior (AS) TM on days 1, 5, 9 and 13 Use of additional interventions (common to all treatment arms): 5 acyclovir 400 tablets per day for a total duration of 6 days and 1 omeprazole capsule per day for 10 days *per protocol analysis |
|
| Outcomes |
Primary outcome measure: proportion of patients whose hearing is improved (criterion of improvement: > 10 dB decrease in PTA or > 15% in SDS) Secondary outcomes: change in hearing threshold with speech audiometry, concomitant symptoms Primary endpoint for hearing threshold evaluation: 28 days Used PTA: 5PTA (0.25, 0.5, 1, 2, 4 kHz) |
|
| Funding sources | Tehran University of Medical Sciences (TUMS) under Grant No. 90‐03‐48‐15295 | |
| Declarations of interest | None declared | |
| Notes | This study is registered in Iranian Registry for Clinical Trials with code number IRCT201202159039N1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients … divided into … three groups using six‐ block randomization Method." |
| Allocation concealment (selection bias) | Low risk | Quote: "All study personnel, participants, and data analyst except the methodologist were blinded to treatment allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The active and placebo capsules and injection vials … were identical in color, size, weight, and packaging." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The active and placebo capsules and injection vials … were identical in color, size, weight, and packaging." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rate of reported missing outcome data more than 10% (23.8%). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified endpoint measurements of outcome parameters addressed by the review are reported. |
| Other bias | High risk | Treatment delay from onset in patients not reported per group but overall low range of treatment delay among patients. Unexplained very similar outcome of speech tests between "intention‐to‐treat population" and "final population". The term "not meeting criteria" leading to exclusion of further patients is not explained. |
Battaglia 2008.
| Study characteristics | ||
| Methods | Double‐blind, parallel‐group randomised controlled trial with 3 weeks duration of treatment and 7 weeks duration of follow‐up | |
| Participants |
Setting: multicentre trial, USA, January 2004 to January 2006 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment/baseline SDS:
Inclusion criteria: ISSNHL within 6 weeks after onset. Patients with no identifiable cause of sudden hearing loss were considered to have ISSNHL. Exclusion criteria: diagnosis not ISSNHL, pregnancy, receiving previous treatment, Ménière's disease, autoimmune hearing loss, acoustic neuroma or other retrocochlear lesions. History of hearing fluctuation, recent ear infection, surgery or hospitalisation, exposure to ototoxins, trauma, drainage, tinnitus, pain, vertigo or family history of hearing loss. Medical conditions associated with sudden hearing loss such as diabetes, syphilis, chronic renal disease and cardiovascular disease. |
|
| Interventions |
General comparison: intratympanic versus combined versus systemic corticosteroid therapy Intervention group I (n = 17*): "intratympanic therapy": placebo oral + intratympanic injection of dexamethasone, 12 mg/mL, 0.5 to 0.7 mL, 20 min, 3 injections total, 1x per week Intervention group II (n = 16*): "combined therapy": intratympanic injection + oral as in intervention group I and comparator group Comparator group (n = 18*): "systemic therapy": oral 60 mg prednisone with 10 mg taper every 2 days + intratympanic NaCl 0.9% (intratympanic placebo), 3 injections total, 1x per week Use of additional interventions (common to all treatment arms): none *modified intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 43 days Used PTA: 3PTA (0.5, 1, 2 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized to 1 of 3 groups and administered treatment in double‐blinded fashion" Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "administered treatment in double‐blinded fashion". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned but placebo‐controlled, double‐blind study design. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rate of reported missing outcome data more than 10% (15%). |
| Selective reporting (reporting bias) | High risk | Missing standard deviation in reporting of hearing improvement. |
| Other bias | High risk | Recruitment of patients was terminated before number of patients from sample size calculation was reached. Wide range of treatment delay from onset in inclusion criteria. Difference in treatment delay from onset between groups. |
Chang 2010.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with approximately 2 weeks duration of treatment and 20 days duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, China, no dates provided Sample size:
Primary/secondary therapy: secondary therapy after failure of primary therapy Primary therapy: not described Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment/start of initial treatment/start of treatment:
Inclusion criteria: diagnosis of ISSNHL and non‐responsive to conventional therapy within 20 days, resident in the region of Qinghai plateau (> 3000 m over sea level) Exclusion criteria: evidence of retrocochlear and central neuropathy |
|
| Interventions |
General comparison: intratympanic corticosteroid therapy versus no treatment Intervention group (n = 24*): "intratympanic therapy": intratympanic injections of dexamethasone 5 mg/mL, 0.2 to 0.5 mL, 4 to 5 injections in total, 1x every 3 to 4 days Comparator group (n = 24*): "no therapy": none Use of additional interventions (common to both treatment arms): 30 mL Ginkgo biloba, 40 mg ATP, 100 U of coenzyme A intravenous and vitamin B‐complex oral daily *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 20 days Used PTA: 7PTA (0.125, 0.25, 0.5, 1, 2, 4, 8 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement. For the meta‐analysis it was assumed that the number of patients analysed per treatment arm is identical to the respective randomised number of patients per treatment arm. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Treatment delay from onset to secondary treatment in patients not reported. |
Choi 2011.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 15 days duration of treatment and 8 weeks duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Republic of Korea, August 2008 to January 2010 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment:
Inclusion criteria: diagnostic criteria for SSNHL were the acute onset of hearing loss of 30 dB or more over at least 3 contiguous audiometric frequencies, which may have occurred within 3 days. The treatments were initiated within 7 days after onset.Exclusion criteria: those with medical or central nervous system conditions, including syphilis, chronic renal disease, cardiovascular disease and retrocochlear lesions were excluded from the study. Those with true whirling type vertigo, family history of hearing loss, history of fluctuating hearing loss, head trauma and otologic surgery were also excluded from the investigation. |
|
| Interventions |
General comparison: intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids alone Intervention group (n = 19*): "combined therapy": intratympanic injection of dexamethasone, 5 mg/mL, 0.3 mL, 1x per day for 5 consecutive days + systemic steroid therapy as in comparator group Comparator group (n = 27*): "systemic therapy": intravenous dexamethasone (10 mg) for 5 days, then oral methylprednisolone for 10 days in tapered doses (48 mg, 40 mg, 32 mg, 24 mg, each for 2 days decreasing by 8 mg every 2 days, and 12 mg for the last 2 days) Use of additional interventions (common to both treatment arms): carbogen (5% CO2, 95% O2) inhalation, low‐salt diet, dextran, pentoxifylline (400 mg), flunarizine (5.9 mg) and stellate ganglion block *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 56 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were divided into 2 different treatment groups on a random basis." Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | High risk | Missing standard deviation in reporting of hearing improvement. Not all pre‐specified follow‐up time points were reported. |
| Other bias | High risk | No sample size calculation performed. Difference in hearing loss before treatment between groups. Unexplained difference in numbers of patients between groups. |
Dispenza 2011.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 3 weeks duration of treatment and 6.7 months duration of follow‐up | |
| Participants |
Setting: tertiary referral hospital, Italy, January 2008 to December 2009 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of treatment:
Inclusion criteria: diagnostic criteria for SSNHL were the acute onset of hearing loss of 30 dB or more over at least 3 contiguous audiometric frequencies, which may have occurred within 24 hoursExclusion criteria: previous episode of hearing loss, history of ear pathology, previous treatments administered elsewhere, retrocochlear lesion, vestibular schwannoma and contraindication to systemic steroid administration |
|
| Interventions |
General comparison: intratympanic versus systemic corticosteroid therapy Intervention group (n = 25*): "intratympanic therapy": intratympanic injection of dexamethasone, 4 mg/mL, "middle ear filled", 4 injections total, 1 x weekly Comparator group (n = 21*): "systemic therapy": oral prednisone, 60 mg, tapering over 14 days Use of additional interventions (common to both treatment arms): none *per protocol analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 204 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | Quote: "The Authors … declare that any financial support was obtained by private organization" (private organisation was not further specified). | |
| Declarations of interest | Quote: "The Authors have not conflict of interest with organization cited in the study …" (no organisation was cited in the publication). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly divided in two groups according to treatment". Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Among 51 patients … we evaluated 46 patients that completed the protocol." Missing outcome data per treatment arm not reported. Moderate missing outcome data (5% to 10%) were probably not balanced across treatment arms (9.8%). |
| Selective reporting (reporting bias) | High risk | Not all pre‐specified follow‐up time points were reported. |
| Other bias | High risk | No sample size calculation performed. Difference in both treatment delay from onset and hearing loss before treatment between groups. Standard deviation for both treatment delay from onset and hearing loss before treatment not reported. |
Ermutlu 2017.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with approximately 24 days duration of treatment and a 3‐month duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Turkey, June 2013 to January 2014 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment:
Inclusion criteria: unilateral SSHL of at least 30 dB including at least 3 frequencies and occurring within 72 h. Additional inclusion criteria were as follows: 1) age between 18 and 80 years, 2) time prior to treatment not exceeding 7 days and 3) no history of previous treatment Exclusion criteria: identifiable cause of hearing loss, a history of previous otologic surgery on the affected ear, an acute or chronic otitis media of the affected ear, retrocochlear pathology |
|
| Interventions |
General comparison: intratympanic versus systemic corticosteroid therapy Intervention group (n = 19*): "intratympanic therapy": intratympanic 0.5 to 0.7 cc dexamethasone (8 mg/2 mL) 3 times every other day Comparator group (n = 16*): "systemic therapy": oral 1 mg/kg (maximum 80 mg) prednisolone and tapering 10 mg every 3 days Use of additional interventions (common to both treatment arms): intravenous low molecular weight dextran (5 cc/kg) for 5 to 10 days, oral acetazolamide for a month, oral acyclovir for 5 days, oral betahistine and oral trimetazidine for 3 months *per protocol analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 90 days Used PTA: 3PTA (0.5, 1, 2 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | None declared | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided into oral steroid (OS) and intratympanic steroid (ITS) groups". Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rate of reported missing outcome data more than 10% (14.6%). |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. |
Gundogan 2013.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 14 days duration of treatment and 4 weeks duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Turkey, December 2009 to January 2013 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of treatment/baseline SDS:
Inclusion criteria: 1) unexplained sudden sensorineural hearing loss, which was defined as a sensorineural hearing loss of at least 30 dB at 3 contiguous frequencies over a period of ≤ 3 days; 2) time from the onset of hearing loss to the treatment of ≤ 14 days; 3) no initial treatment before; 4) no history of ear disease in the affected ear; 5) and unilateral sudden hearing lossExclusion criteria: chronic otitis media, trauma, previous radiotherapy or chemotherapy, recent use of ototoxic drugs, liver or renal dysfunction, retrocochlear lesion and interval to first treatment greater than 14 days from onset |
|
| Interventions |
General comparison: intratympanic plus systemic corticosteroids (combined) versus systemic corticosteroids Intervention group (n = 37*): "combined therapy": intratympanic injection of methylprednisolone, 62.5 mg/mL, 0.4 mL, 4 injections total, 1x every 3 days + systemic steroid therapy as in comparator group Comparator group (n = 36*): "systemic therapy": oral methylprednisolone, 1 mg per kg, 10 mg taper every 3 days Use of additional interventions (common to both treatment arms): none *per protocol analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 28 days Used PTA: 4PTA (0.5, 1, 2, 3 kHz) |
|
| Funding sources | None declared | |
| Declarations of interest | None declared | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to 2 groups according to treatment ", "The blocked randomization was used in this study". |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported moderate missing outcome data (5% to 10%) balanced in numbers across intervention groups (7.6%). |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Notable difference in speech discrimination scores between groups before start of therapy. |
Ho 2004.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 15 days duration of treatment and 1.5‐month duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Taiwan, January 2001 to June 2003 Sample size:
Primary/secondary therapy: secondary therapy after failure of primary therapy Primary therapy: methylprednisolone oral 1 mg/kg per day for 5 days, followed by tapering to 10 mg/day for another 5 days plus vasodilators (nicametate citrate, 3x per day), vitamin B‐complex and fludiazepam (0.25 mg 3x per day) and carbogen inhalation therapy (95% O2 and 5% CO2) Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of initial treatment/start of treatment:
Inclusion criteria: 1) unilateral severe or profound idiopathic sudden SSNHL, 2) HL occurring within 24 hours, 3) no otologic history in the affected ear, and 4) hearing improvement < 30 dB in PTA, no recovery of hearing, or worsening of hearing after primary therapy Exclusion criteria: mumps, measles, rubella, cytomegaly viruses and retrocochlear lesion |
|
| Interventions |
General comparison: intratympanic corticosteroid therapy versus no treatment Intervention group (n = 15*): "intratympanic therapy": intratympanic injection of dexamethasone, 4 mg/mL, 0.4 to 0.7 mL, 3 injections total, 1x per week Comparator group (n = 14*): "no therapy": continuation of initial oral "standard therapy" without corticosteroids: vasodilators (nicametate citrate, 3 times a day orally), vitamin B‐complex and benzodiazepine (fludiazepam, 0.25 mg 3 times a day orally) Use of additional interventions (common to both treatment arms): none *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 49 days Used PTA: 6PTA (0.25, 0.5, 1, 2, 4, 8 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients … were further randomly assigned". Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | High risk | Not all pre‐specified follow‐up time points were reported. |
| Other bias | High risk | No sample size calculation performed. Mean of hearing threshold before treatment in control group not reported. Treatment delay from onset to secondary treatment in patients not reported but reported treatment delay to initial treatment and duration of initial treatment. Possible difference in treatment delay from onset between groups. |
Hong 2009.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 8 days duration of treatment and 3‐month duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Republic of Korea, May 2007 to January 2009 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of treatment:
Inclusion criteria: ISSNHL of 30 dB or more with over 3 contiguous audiometric frequencies that occurred in fewer than 3 days Exclusion criteria: ISSNHL with vertigo, diabetes, Ménière’s disease, tumours, treatment onset of more than 15 days |
|
| Interventions |
General comparison: intratympanic versus systemic corticosteroid therapy Intervention group (n = 32*): "intratympanic therapy": intratympanic injection of dexamethasone, 5 mg/mL, 0.3 to 0.4 mL, 8 injections total, 1 x per day Comparator group (n = 31*): "systemic therapy": oral prednisolone 60 mg per day for 4 days, 40 mg per day for 2 days and 20 mg per day for 2 days Use of additional interventions (only comparator group):"peripheral vasodilator" and Ginkgo biloba extract *per protocol analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 90 days Used PTA: 4PTA (0.5, 1, 2, 3 kHz) |
|
| Funding sources | Grant (code # 200810FTH010103002) from BioGreen21 Program, Rural Development Administration, Republic of Korea | |
| Declarations of interest | None declared | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "IT dexamethasone or oral prednisolone, was assigned for patients with ISSHL alternatively and randomly." Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "outcome assessors were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rate of reported missing outcome data more than 10% (16%). |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Standard deviation of treatment delay from onset not reported. |
Huang 2021.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group, quasi‐randomised controlled trial with 12 days duration of treatment and follow‐up on day 13 | |
| Participants |
Setting: not stated (secondary care setting likely, due to author affiliations), China, January 2013 to October 2018 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment:
Inclusion criteria: onset of SSNHL within 72 hours with hearing loss of 30 dB or greater for 3 consecutive frequencies by pure tone threshold audiometry (PTA 0.25, 0.5, 1, 2, 4 and 8 kHz) Exclusion criteria: aged under 18 or over 65 years, unmanaged serious systemic diseases, diabetes or hypertension, epilepsy, psychosis, active ulcer of digestive tract or any contraindications for hormone therapy, identifiable cause of hearing loss (such as radiation, noise, acute or chronic otitis media, Ménière's disease, autoimmune diseases, large vestibular aqueduct syndrome or retrocochlear disease) |
|
| Interventions |
General comparison: intratympanic corticosteroids versus systemic corticosteroids Intervention group (n = 49*): intratympanic injection of dexamethasone, 1.5 mg/0.3 mL, once every other day for 24 days Comparator group (n = 49*): intravenous dexamethasone, 10 mg per day on days 1 to 4, 7.5 mg per day on days 5 to 8 and 5 mg per day on days 9 to 12, followed by intratympanic dexamethasone once every alternate day for 12 days *per protocol analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcome measure:
Primary endpoint for hearing threshold evaluation: 90 days |
|
| Funding sources | National Natural Science Foundation of China | |
| Declarations of interest | Authors report no conflicts of interest with regard to the manuscript | |
| Notes | Participants who initially received systemic dexamethasone for 12 days subsequently received intratympanic dexamethasone. Therefore endpoint data are not relevant for this review, but interim data reported on day 12 were used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were alternately divided into group A or group B according to the time of admission" |
| Allocation concealment (selection bias) | High risk | Quote: "Patients were alternately divided into group A or group B according to the time of admission" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description regarding whether outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout less than 10% and balanced in both groups. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported fully according to the methods of the paper. No trial protocol available to assess further. |
| Other bias | High risk | No sample size calculation performed. Included data are available only at an early‐test point (day 13) and may not adequately represent longer‐term results of the treatment. Treatment delay from onset in patients not reported. |
Koltsidopoulos 2013.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 9 days duration of treatment and 3‐month duration of follow‐up | |
| Participants |
Setting: multicentre trial, Greece, November 2009 to January 2012 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment/baseline SDS:
Inclusion criteria: 1) sudden sensorineural hearing loss of unknown cause greater than 30 dB in 3 contiguous audiometric frequencies developing within 3 days, 2) time from onset of hearing loss to treatment administration of 20 days or less, 3) no otologic history in the affected ear, 4) ≥ 18 years Exclusion criteria: mumps, toxoplasmosis, borreliosis, HIV, CMV, HSV, measles, rubella, influenza virus, syphilis, retrocochlear pathology |
|
| Interventions |
General comparison: intratympanic plus systemic corticosteroids (combined therapy) versus systemic corticosteroids Intervention group (n = 46*): "combined therapy": intratympanic injection of dexamethasone, 4 mg/mL, 0.4 to 0.6 mL, 3 injections total, 1 x every 2 days + systemic therapy as in comparator group Comparator group (n = 46*): "systemic therapy": intravenous prednisolone, 75 mg/d for 3 days, followed by 50 mg/d for the next 3 days and 25 mg/d for another 3 days Use of additional interventions (common to both treatment arms): none *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 100 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) and 7PTA (0.25, 0.5, 1, 2, 4, 6, 8 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were allocated to 2 groups on a 1:1 basis, depending on the odd or even number of presentation." |
| Allocation concealment (selection bias) | High risk | No allocation concealment possible because of the method of random sequence generation used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "audiologic assessment and data analysis were kept blinded to the allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all patients complied with the follow up protocol." |
| Selective reporting (reporting bias) | High risk | Hearing improvement only reported as median but mean pre‐specified as outcome parameter. Both hearing loss before treatment and hearing improvement is only provided by a 4PTA but not by a 7PTA although the latter is the primary outcome parameter. |
| Other bias | Low risk | No indications of other bias. |
Kosyakov 2011.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 6‐month duration of treatment and 6‐month duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Russia, no dates provided Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment:
Inclusion criteria: SSNHL with hearing loss in 3 contiguous frequencies of at least 30 dB, who had not previously been treated and were at least 18 years old; treatment delay less than 1 monthExclusion criteria: 1) patients with somatic pathology (such as diabetes, hypertension, gastric ulcer, tuberculosis, glaucoma, etc.), for whom systemic steroids were contra‐indicated; 2) oncology patients; 3) patients with autoimmune diseases or those who were constantly or periodically taking steroids; 4) patients who were or have been taking ototoxic agents; 5) patients with acoustic neurinoma; 6) pregnant and nursing women; 7) patients with middle ear diseases, abnormal type of tympanometric curves or barotrauma in their anamnesis; 8) those who had intolerance for any component of treatment; 9) those who had SSNHL in the only hearing ear |
|
| Interventions |
General comparison: intratympanic versus systemic corticosteroid therapy Intervention group I (n = 24*): "intratympanic therapy": intratympanic injection of dexamethasone, 4 mg/mL (volume not specified), every day for 10 days, then every other day over 20 days, then 2 times a week over further 5 months Comparator group I (n = 24*): "systemic therapy": intravenous dexamethasone 0.1 mg/kg daily for 10 days, followed by a decreasing dose over 5 days Comparator group II (n = 25*): "systemic therapy": intravenous dexamethasone 0.1 mg/kg daily for 10 days, followed by a decreasing dose over 5 days Use of additional interventions (only used in comparator group I): pentoxifylline, cocarboxylase, potassium and magnesium aspartate intravenously and vitamin B‐complex intramuscularly *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 183 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | Intratympanic treatment was administered over 6 months, which is much longer than all other intratympanic treatment protocols in this review. Also, the duration of intratympanic and systemic therapy differed considerably, intratympanic therapy lasting 6 months and systemic therapy lasting only 15 days. We therefore investigated whether the inclusion of this study had a notable impact on the overall results by conducting a sensitivity analysis in which it was excluded. Only the group receiving systemic corticosteroid without additional treatment was included as a comparator. This was because the additional treatments were not received by the group receiving intratympanic corticosteroid. This study was included in meta‐analyses despite a unit of analysis of ears instead of patients, as only 2 patients with bilateral hearing loss were included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from protocol: "The randomisation will be done using a random number table of the software Statistica (StatSoft Inc., version 6.1)." |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from protocol: "Audiologists producing hearing loss assessment will be blinded". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcome criteria are pre‐specified in the later sent protocol and reported in the results of the publication. |
| Other bias | High risk | No sample size calculation performed. Treatment delay from onset in patients not reported. Only mild cases of ISSNHL included. Wide range of treatment delay from onset in inclusion criteria. Some participants (n = 2) had bilateral disease, but analysis does not account for correlation between ears. Duration of treatment longer in intervention arm (6 months) than in the comparator arm (15 days). |
Lee 2011.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group, randomised controlled trial with 2 weeks duration of treatment and 8 weeks duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Republic of Korea, March 2004 to December 2007 Sample size:
Primary/secondary therapy: secondary therapy after failure of primary therapy Primary therapy: oral steroids 60 mg/day for 5 days, followed by tapering for 5 days Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of initial treatment/start of treatment:
Inclusion criteria: SSNHL with more than 30 dB HL in 3 serial frequency lasting from 12 h to several days. Failure in initial therapy (≤ 10 dB decrease in PTA after primary therapy)Exclusion criteria: medical or central diseases such as diabetes, hypertension, autoimmune disorders, syphilis, acoustic schwannoma and others that may affect hearing recovery |
|
| Interventions |
General comparison: intratympanic corticosteroid therapy versus no treatment Intervention group I (n = 21*): "intratympanic therapy": intratympanic injection of dexamethasone, 5 mg/mL, 0.3 to 0.4 mL, 4 injections total, 2 x per week Comparator group (n = 25*): "no therapy": none Use of additional interventions (common to both treatment arms): none *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 56 days Used PTA: 4PTA (0.5, 1, 2, 3 kHz) |
|
| Funding sources | Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF‐2006‐E00081) | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This case‐controlled study was prospectively designed", "Forty‐six patients were randomly classified into two groups" Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | High risk | Missing standard deviation in reporting of hearing improvement. Not all pre‐specified follow‐up time points were reported. |
| Other bias | High risk | No sample size calculation performed. Unexplained difference in numbers of patients between groups. Treatment delay from onset to secondary treatment in patients not reported, but reported treatment delay to initial treatment and duration of initial treatment. |
Li 2011.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 15 days duration of treatment and 2‐month duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, China, July 2006 to September 2009 Sample size:
Primary/secondary therapy: secondary therapy after failure of primary therapy Primary therapy: 1 mg/kg prednisolone each day for 5 days followed by a division into 4 doses with a gradual tapering over the course of 9 days Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of initial treatment/start of treatment:
Inclusion criteria: 1) SSNHL, which was defined as a sensorineural hearing loss of at least 30 dB at 3 contiguous frequencies over a period of ≤ 3 days; 2) time from the onset of hearing loss to the treatment was ≤ 14 days; 3) no history of ear diseases; 4) no specific causes for the SSNHL after proper investigation; 5) primary therapy (see above); 6) average of PTA was < 30 dB for the affected ear or < 10 dB from the contralateral ear at the end of primary treatment Exclusion criteria: 1) bilateral hearing loss; 2) other contraindications to the administration of intratympanic (IT) steroids; 3) the presence of a neoplasm or recent chemotherapy or radiation therapy; 4) congenital cochlear malformations or the presence of otitis media with an abnormal tympanogram; 5) recent use of ototoxic medications; 6) liver or renal dysfunction, and/or 7) pregnancy |
|
| Interventions |
General comparison: intratympanic corticosteroids versus ear drops or versus no therapy Intervention group (n = 24*): "intratympanic therapy": intratympanic injection of methylprednisolone, 40 mg/mL, 1 mL, 4 injections total, 2 x every 3 days within 15 days Comparator group I (n = 21*): "ear canal drops": methylprednisolone, 40 mg/mL, 1 mL as drops in ear canal onto intact tympanic membrane, 4 applications total, 1x every 3 days within 15 days Comparator group II (n = 20*): "no treatment": none Use of additional interventions (common to all treatment arms): none *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 60 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | Ministry of Education New Faculty Foundation (20090171120082) and Guangdong Province Medical Science Foundation (B2009075) | |
| Declarations of interest | No information provided | |
| Notes | The treatment arm patients treated by corticosteroid ear canal drops were not included in the meta‐analysis because they cannot be assigned to either the intratympanic or systemic treatment condition in our opinion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[patients] were randomly divided into the IT treatment group (n = 24), the ear drop group (n = 21), and the control group (n = 20)." Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Treatment delay from onset to secondary treatment in patients not reported. Unexplained difference in numbers of patients among groups. |
Lim 2013.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 2 weeks duration of treatment and 2 to 3 weeks duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Republic of Korea, July 2008 to November 2011 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment:
Inclusion criteria: ISSNHL with acute onset of hearing loss greater than 30 dB in 3 consecutive frequencies occurring within 3 daysExclusion criteria: acoustic trauma, barotrauma, Ménière’s disease, tumour disease, autoimmune disease, infection |
|
| Interventions |
General comparison: intratympanic versus combined versus systemic corticosteroid therapy Intervention group I (n = 20*): "intratympanic therapy": intratympanic injection of dexamethasone 5 mg/mL, 0.3 to 0.4 mL, 4 injections total, 2x per week Intervention group II (n = 20*): "combined therapy": treatment as in intervention group I and comparator group combined Comparator group (n = 20*): "systemic therapy": oral prednisolone, 60 mg per day for 5 days, 40 mg per day for 2 days, 20 mg per day for 2 days and 10 mg per day for 1 day Use of additional interventions (common to all treatment arms): none *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: intervention groups: 21 days, control group: 17 days Used PTA: 4PTA (0.5, 1, 2, 3 kHz) |
|
| Funding sources | None declared | |
| Declarations of interest | None declared | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "method of randomization is a consecutive allocation by visit sequence." |
| Allocation concealment (selection bias) | High risk | No allocation concealment possible because of the method of random sequence generation used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "patient treatment condition were blinded only to outcome assessors" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | Difference in time of treatment delay from onset between groups. Longer follow‐up time in the intervention groups (27 days) than in the control group (17 days). |
Peng 2008.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 10 or 20 days duration of treatment and a 17 or 27 days duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, China, 2005 to 2007 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of treatment:
Inclusion criteria: unilateral ISSNHL Exclusion criteria: middle ear or retrocochlear disease, previous history of hearing loss, previously treatment, age over 65 years |
|
| Interventions |
General comparison: intratympanic corticosteroids versus injection of corticosteroids through pharyngotympanic tube versus systemic corticosteroids by intravenous and oral administration Intervention group I (n = 21*): "intratympanic therapy": intratympanic injections of dexamethasone 5 mg, 10 injections in total, 1x every day for 10 days Intervention group II (n = 21*): "injection through pharyngotympanic tube": injections of dexamethasone 5 mg with pharyngotympanic tube catheter, 10 injections in total, 1x every day for 10 days Comparator group I (n = 21*): "systemic therapy i.v.": dexamethasone intravenous 10 mg per day for 4 days; after 4 days 5 mg per day for 6 days Comparator group II (n = 21*): "systemic therapy p.o.": dexamethasone oral 0.75 mg, 3 times per day; after 7 days, 0.75 mg, 2 times per day for 3 days Use of additional interventions (common to all treatment arms): intravenous low molecular dextran 500 mL, buflomedil 0.2 g, energy mixture. Intramuscular injection of vitamin B1 0.1 g, B12 0.5 mg, once a day for 10 days Patients underwent 1 or 2 treatment cycles depending on pure tone audiometry after the first treatment cycle. *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 17 or 27 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | Data from the oral systemic treatment arm were included in the review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation according to date of visit of patients. |
| Allocation concealment (selection bias) | High risk | No allocation concealment possible because of the method of random sequence generation used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Wide range of treatment delay from onset among included patients. Different time of treatment (10 or 20 days) and follow‐up (17 or 27 days) between patients within groups. |
Plontke 2009.
| Study characteristics | ||
| Methods | Triple‐blinded, parallel‐group randomised controlled trial with 2 weeks duration of treatment and 2 weeks duration of follow‐up | |
| Participants |
Setting: multicentre trial, Germany, June 2003 to March 2006 Sample size:
Primary/secondary therapy: secondary therapy after failure of primary therapy Primary therapy: intravenous prednisolone 250 mg/day for 3 days, followed by a dose reduction of 50% every 2 days together with systemic rheological medication (pentoxifylline, 3 x 400 mg/day) and an antioxidant drug (alpha lipoic acid, 1 x 600 mg/day) Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of initial treatment/start of treatment/baseline SRT/baseline SD (max):
Inclusion criteria: 1) age between 18 and 75, 2) diagnosis of sudden (occurring within 72 hours), unilateral, sensorineural hearing loss (ISSNHL) between 12 and 21 days before randomisation, 3) hearing threshold of ≥ 50 dB HL for 3 or more frequencies in standard pure tone, air‐conducted audiogram within the range of 0.5 to 4 kHz (0.5, 1, 2, 3 and 4 kHz), ≥ 60 dB for 2 or ≥ 70 dB HL for any frequency within this range, or a speech reception threshold of ≥ 70 dB SPL or a speech discrimination score of ≤ 30%, 4) insufficient recovery of hearing after systemic standard therapy, that is, a hearing threshold in the contralateral ear of at least 20 dB HL better than the affected ear in at least 3 frequencies between 0.5 to 4 kHz in addition to 3) Exclusion criteria: 1) middle or external ear disease; 2) conductive hearing loss ≥ 10 dB; 3) bilateral ISSNHL; 4) acute hearing loss other than ISSNHL, for example, acoustic trauma, Ménière's disease, fluctuating hearing loss, endolymphatic hydrops, suspected retro‐cochlear lesion, hearing loss after ear surgery, perilymphatic fistula or barotraumas; 5) ototoxic treatment such as chemotherapy or loop diuretics; 6) history of an ischaemic disorder (stroke, heart attack, peripheral arterial occlusion disease) or autoimmune disease; 7) any severe psychiatric or neurological disease (e.g. epilepsy, Parkinson’s disease, dementia/Alzheimer’s disease, suspected neuroborreliosis, multiple sclerosis) |
|
| Interventions |
General comparison: intratympanic corticosteroid therapy versus placebo Intervention group (n = 10*): "intratympanic therapy": continuous intratympanic application of dexamethasone by round window catheter, 4 mg/mL, total intratympanic volume of 2.016 mL over 14 days Comparator group (n = 10*): "placebo": continuous intratympanic application of NaCl/0.9% by round window catheter (placebo), total intratympanic volume of 2.016 mL over 14 days Use of additional interventions (common to both treatment arms): none *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 14 days Used PTA: 4PTA (0.5, 1, 2, 3 kHz) |
|
| Funding sources | State Ministry of Baden‐Wuerttemberg for Sciences, Research and Arts (Germany) through University of Tübingen, Germany | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator was used (not mentioned in paper, but the protocol is known). |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug containers of identical appearance (not mentioned in paper, but the protocol is known). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and study personnel were blinded to treatment (placebo‐controlled). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded (placebo‐controlled). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported moderate missing outcome data (5% to 10%) balanced in numbers across intervention groups (8.6%). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified endpoint measurements of outcome parameters addressed by the review are reported. |
| Other bias | High risk | Early endpoint of 14 days after start of treatment only. |
Qu 2015.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 10 days duration of treatment and a 10 days duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, China, July 2012 to July 2014 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of treatment:
Inclusion criteria: diagnosis of ISSNHL, age 18 to 65 years; onset was less than 2 weeks and no previously treatment Exclusion criteria: family history of deafness, previous history of otitis media, external or middle ear disease, ototoxic drug use or long‐term exposure to noise |
|
| Interventions |
General comparison: intratympanic corticosteroids versus post auricular injection of corticosteroids and systemic corticosteroids Intervention group (n = 57*): "intratympanic therapy": intratympanic injections of methylprednisolone 40 mg, 5 injections in total, 1 x every 2 days for 10 days Comparator group I (n = 62*): "post auricular injection": post auricular injection of methylprednisolone 40 mg, 5 injections in total, 1 x every 2 days for 10 days Comparator group II (n = 69*): "systemic therapy": methylprednisolone 80 mg intravenous once a day, after 4 days reduced to 40 mg once a day for 3 days Use of additional interventions (common to all treatment arms): vasodilators, neurotropic drugs, anticoagulant drugs for 10 days *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 10 days Used PTA: information not provided |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised after random number table. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Treatment delay from onset to initial therapy in patients not reported. Hearing loss before treatment not adequately reported Early endpoint of 10 days after start of treatment only. Unexplained difference in number of patients between treatment arms. |
Rauch 2011.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 2 weeks duration of treatment and a 6‐month duration of follow‐up | |
| Participants |
Setting: multicentre trial, USA, December 2004 to October 2009 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of treatment/baseline WRS:
Inclusion criteria: age > 18 years, unilateral SNHL that developed within 72 hours and was present for < 14 days, PTA > 50 dB and the affected ear must have been > 30 dB worse than the contralateral ear in at least 1 of the 4 PTA frequencies, to the best of the participant’s knowledge, hearing must have been symmetric prior to onset of SSNHL Exclusion criteria: structural or retrocochlear pathology, participants were neither known nor expected to have had any preceding otolaryngological encounters |
|
| Interventions |
General comparison: intratympanic versus systemic corticosteroid therapy Intervention group (n = 129*): "intratympanic therapy": intratympanic 40 mg/mL of methylprednisolone, 1 mL, 1x every 3 to 4 days over 2 weeks Comparator group (n = 121*): "systemic therapy": oral 60 mg/d for 14 days, followed by a 5‐day taper (50 mg, 40 mg, 30 mg, 20 mg and to 10 mg), for a total of 19 days Use of additional interventions (common to both treatment arms): none *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 60 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | Quote: "This study was funded by grant U01‐DC006296 from the National Institute on Deafness and Communication Disorders (USA)." | |
| Declarations of interest | Quote: "All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Harris reported that he has a financial interest in Otonomy Inc. Drs Rauch, Carey, Gantz, and Harris reported that they have served as paid consultants of Otonomy, and Drs Rauch, Gantz, and Linstrom reported that they have been investigators in a clinical trial supported by Otonomy Inc; otherwise, no other conflicts were reported." | |
| Notes | This study is registered in clinicaltrials.gov (NCT00097448) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Permuted block randomization stratified by study site … was accomplished by telephone call to the data coordinating center." "The randomization codes were computer generated … Only personnel at the data coordinating center had access to the codes" |
| Allocation concealment (selection bias) | Low risk | Quote: "Permuted block randomization stratified by study site … was accomplished by telephone call to the data coordinating center." "The randomization codes were computer generated … Only personnel at the data coordinating center had access to the codes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The participants and treating physicians were not blinded to treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Audiologists were blinded to treatment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low missing outcome data (< 5%). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified endpoint measurements of outcome parameters addressed by the review are reported. |
| Other bias | Low risk | No indication of other bias. |
Rupasinghe 2017.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 15 days duration of treatment and 3‐month duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Sri Lanka, September 2013 to August 2015 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment:
Inclusion criteria: 1) patients with a hearing loss of more than 10 dB HL in 3 consecutive frequencies; 2) patients presenting within 2 weeks of onset of hearing loss; 3) patients with diabetes mellitus were included with a referral to the resident physician regarding glycaemic control Exclusion criteria: 1) patients who have already been diagnosed and treated; 2) patients on ototoxic medication |
|
| Interventions |
General comparison: intratympanic versus systemic corticosteroid therapy Intervention group (n = 17*): "intratympanic therapy": intratympanic dexamethasone 0.3 to 0.5 mL and weekly up to 3 doses Comparator group (n = 20*): "systemic therapy": oral prednisolone 60 mg per day for 5 days Use of additional interventions (common to both treatment arms): none *per protocol analysis (at 1‐month follow‐up) |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 90 days Used PTA: not specified |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | The follow‐up time point at 1 month was included in the meta‐analysis instead of 3 months because of a high loss to follow‐up rate at the 3‐month time point | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "[Patients] were alternatively allocated to the two treatment groups according to the register." |
| Allocation concealment (selection bias) | High risk | No allocation concealment possible because of the method of random sequence generation used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rate of reported missing outcome data more than 10% (35.1%). |
| Selective reporting (reporting bias) | High risk | Criteria for hearing improvement in patients not reported. |
| Other bias | High risk | No sample size calculation performed. Patients with very mild hearing loss included (> 10 dB HL). Hearing threshold before treatment not reported. Treatment delay from onset in patients not adequately reported. Difference in treatment delay from onset in patients between groups. Single authorship generally exhibits a certain risk of bias. |
Swachia 2016.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 2 weeks duration of treatment and 2‐month duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, India, dates not provided Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of treatment:
Inclusion criteria: > 18 years, SSNHL meeting NIDCD criteria, occurred within a course of 14 daysExclusion criteria: prior history of ear disease, history of noise‐induced trauma, congenital hearing loss, pregnancy, contraindication for steroids, history of head and neck cancer, undergone radiotherapy |
|
| Interventions |
General comparison: intratympanic versus systemic therapy Intervention group (n = 20*): "intratympanic therapy": intratympanic injection of methylprednisolone, 40 mg/mL, 4 injections total, 2 times per week Comparator group (n= 22*): "systemic therapy": oral prednisone 1 mg/kg for 10 days, then 0.5 mg/kg for 2 days, 0.25 mg/kg for 2 days Use of additional interventions (common to both treatment arms): none *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 60 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | None declared | |
| Declarations of interest | Quote: "The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication" (organisation(s) not further specified). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients … were randomly divided into two groups". Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Treatment delay from onset in patients not reported. Unexplained difference in numbers of patients between groups. |
Tong 2021.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 10 days duration of treatment and 5 weeks duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, China, October 2013 to June 2014 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of treatment:
Inclusion criteria: 1) unilateral ISSNHL of > 20 dB hearing level (HL) in least 3 consecutive frequencies, that occurred within ≤ 3 days without any identifiable cause, 2) a type A tympanogram, 3) a normal or almost‐normal HL in the contralateral ear (a 6‐frequency pure tone average [PTA] of < 30 dB HL), 4) an interval from the onset of symptoms to the beginning of therapy of < 14 days, 5) no history of disease in the affected ear, 6) no previous treatment, 7) age ≥ 18 yearsExclusion criteria: 1) the presence of a retrocochlear lesion or a neoplasm, 2) a history of chronic otitis media in the affected ear, 3) the presence of congenital cochlear malformations, 4) inadequate follow‐up after treatment (i.e. < 4 weeks), 5) recent use of ototoxic medications, 6) pregnancy, 7) a history of genetic sensorineural hearing loss, and 8) reported acoustic trauma or barotrauma |
|
| Interventions |
General comparison: intratympanic versus systemic corticosteroid therapy Intervention group I (n = 30*): "intratympanic therapy": intratympanic 0.6 mL of 40 mg/mL methylprednisolone every second day for 10 days Comparator group I (n = 30*): "systemic therapy oral": oral methylprednisolone 0.8 mg/kg/day for the first 5 days and 8 mg/day for the next 5 days. Comparator group II (n = 30*): "systemic therapy intravenous": intravenous methylprednisolone 0.8 mg/kg/day for the first 5 days and 8 mg/day for the next 5 days. Use of additional interventions (common to all treatment arms): none *per protocol analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 38 days Used PTA: 6PTA (0.25, 0.5, 1, 2, 4, 8 kHz) |
|
| Funding sources | The study was supported by the Education Department of Anhui Province (project No. 2014KJ: 120) | |
| Declarations of interest | None declared | |
| Notes | Only the treatment arm with oral corticosteroids was included in the meta‐analysis as the comparator to the intratympanic treatment arm because systemic groups in most of other studies were treated with oral corticosteroids. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The randomization technique was a consecutive allocation by the visit sequence." |
| Allocation concealment (selection bias) | High risk | No allocation concealment possible because of the method of random sequence generation used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The outcome assessors were blinded as to the treatment method used". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported moderate missing outcome data (5% to 10%) balanced in numbers across intervention groups (6.3%). |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Criteria for successful treatment was defined as > 30 dB HL decrease of PTA but used was > 10 dB HL decrease. |
Tsounis 2018.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 10 days duration of treatment and 3‐month duration of follow‐up | |
| Participants |
Setting: multicentre trial, Greece, September 2013 to September 2016 Sample size:
Primary/secondary therapy: primary therapy Participant (baseline) characteristics: age/sex (male, female)/hearing loss at start of therapy/start of treatment:
Inclusion criteria: 18 years or older, ISSNHL with minimum 30 dB HL hearing loss in 3 consecutive octaves that had occurred within a course of 3 days, hearing thresholds of the affected frequencies must have been 55 dB HL or higher, the affected ear must have been at least 30 dB HL worse than the contralateral ear in at least 1 of the affected frequencies, symmetric hearing prior to onset of sensorineural hearing loss, type A tympanogram Exclusion criteria: any recognised cause of SSHL such as Ménière's disease, acoustic neuroma or other retrocochlear lesions, any previous treatment for the specific episode of ISSNHL, interval of more than 14 days from the onset of the disease to initiation of the treatment and any contraindication to the use of systemic steroids, such as uncontrolled diabetes mellitus or hypertension. Conductive or mixed hearing loss. |
|
| Interventions |
General comparison: intratympanic versus combined versus systemic corticosteroid therapy Intervention group I (n = 34*): "intratympanic therapy": intratympanic 0.4 to 0.6 mL of 62.5 mg/mL methylprednisolone on day 1, 3, 5, 10 Intervention group II (n = 33*): "combined therapy": intratympanic injection + intravenous as in intervention group I and comparator group Comparator group (n = 35*): "systemic therapy": intravenous 1 mg/kg prednisolone per day for 7 days followed by 0.5 mg/kg per day for 3 days, continuing with oral 32 mg/day methylprednisolone for 4 days followed by 16 mg/day for 3 days Use of additional interventions (common to all treatment arms): none *per protocol analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 90 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | None declared | |
| Notes | This study is registered in the international scientific database Australian New Zealand Clinical Trials Registry (ANZCTR, ID: ACTRN12613001032741) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was accomplished by generating sequential random numbers (sequential randomization) using a computer‐based software." |
| Allocation concealment (selection bias) | Low risk | Quote: "The random numbers were placed in closed envelopes and were given sequentially to every patient that was recruited." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Treating physicians and patients were aware of the allocated arm." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The physicians that performed the audiologic assessment and data analysis were kept blinded to the allocation." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rate of reported missing outcome data more than 10% (15%). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified endpoint measurements of outcome parameters addressed by the review are reported. |
| Other bias | Low risk | No indication of other bias. |
Wu 2011.
| Study characteristics | ||
| Methods | Triple‐blinded, parallel‐group, randomised controlled trial with 14 days duration of treatment and 1‐month duration of follow‐up | |
| Participants |
Setting: multicentre trial, China, October 2007 to September 2008 Sample size:
Primary/secondary therapy: secondary therapy after failure of primary therapy Primary therapy: 5 days of an intravenous steroid therapy with Solu‐Medrol 40 mg every 12 h, plus 5 days of tapering with oral prednisolone starting from a daily divided dose of 1 mg/kg Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of initial treatment/start of treatment:
Inclusion criteria: 1) sudden unilateral sensorineural hearing loss (occurring within 72 h) of greater than 30 dB in at least 3 contiguous frequencies, 2) normal or nearly normal hearing in the better ear (4‐frequency pure tone average (PTA) < 30 dB), 3) currently receiving systemic steroid therapy that started within 7 days of SSHL onset, 4) a post‐systemic therapy PTA difference between impaired and healthy ears of greater than 20 dB, 5) a type A tympanogram and 6) older than 18 years Exclusion criteria: 1) the presence of a neoplasm or retrocochlear lesion, 2) the presence of congenital cochlear malformations, 3) the presence of otitis media, 4) the presence of other neurologic disorders, 5) recent use of ototoxic medications, 6) liver or renal dysfunction and 7) pregnancy |
|
| Interventions |
General comparison: intratympanic corticosteroids versus placebo Intervention group (n = 27*): "intratympanic therapy": intratympanic injection of dexamethasone, 4 mg/mL, 0.5 mL, 4 injections total, 1x every 4 days Comparator group (n = 28*): "placebo": intratympanic injection of normal saline, 0.5 mL, 4 injections total, 1x every 4 days Use of additional interventions (common to both treatment arms): none *per protocol analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 30 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "independent physician used a computer‐generated randomisation schedule". |
| Allocation concealment (selection bias) | Low risk | Quote: "computer‐generated randomisation list was given to the pharmacy departments". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The principal physician was blind to the drugs, …. The subjects were blinded to the drugs." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "…and the audiologists were blind to the subjects". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reported moderate missing outcome data (5% to 10%) was related to the advised treatment arm (8.3%). |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Treatment delay to secondary therapy in patients not reported. |
Xenellis 2006.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 2 weeks duration of treatment and 2‐month duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, Greece, no dates provided Sample size:
Primary/secondary therapy: secondary therapy after failure of primary therapy Primary therapy: prednisolone intravenous, 1 mg/kg per day for 10 days, divided in 3 doses, gradually tapered for 5 days. Acyclovir, 4 g/day for 5 days, divided in 5 doses. Buflomedil hydrochloride, 300 mg, divided in 3 doses, for 10 days. Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of initial treatment/start of treatment:
Inclusion criteria: 1) SSHL, defined as a sensorineural hearing loss of at least 30 dB in 3 contiguous frequencies over a period of 3 days or less, 2) time period from onset of hearing loss to treatment administration of 30 days or less, 3) no history of ear disease, 4) no specific cause for the SSHL after proper investigation, 5) pure tone 4‐frequency (0.5, 1, 2 and 4 KHz) average (PTA) worse than 30 dB or worse than 10 dB from the contralateral ear at the end of primary treatmentExclusion criteria: not mentioned |
|
| Interventions |
General comparison: intratympanic corticosteroids versus no therapy Intervention group (n = 19*): "intratympanic therapy": intratympanic injection of methylprednisolone, 40 mg/mL, 4 injections total, 1x every 4 days Comparator group (n = 18*): "no treatment": none Use of additional interventions (common to both treatment arms): none *intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 60 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomized 1:1 into IT treatment or control group." Method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label trial, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting in the outcome parameters addressed by the review. |
| Other bias | High risk | No sample size calculation performed. Wide range of treatment delay from onset among included patients. Treatment delay from onset to secondary treatment in patients not reported but reported treatment delay to initial treatment (without standard deviation) and duration of initial treatment. Possible difference in treatment delay from onset between groups. Completely equal baseline PTA is notable. Missing improvement in control group is in contradiction to many other studies but is not discussed. |
Zhou 2011.
| Study characteristics | ||
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 8 days duration of treatment and a 2‐month duration of follow‐up | |
| Participants |
Setting: tertiary referral centre, China, January 2004 to December 2006 Sample size:
Primary/secondary therapy: secondary therapy after failure of primary therapy (after 7 days of initiation of primary treatment) Primary therapy: 125 mg methylprednisolone intravenous for the first day, followed by 32 mg per day oral for 5 days, 16 mg per day for 1 day. Naftidrofuryl 200 mg oral 3 times a day, diazepam 5 mg oral 3 times a day and low molecular weight heparin 0.4 mL subcutaneous 2 times day or low molecular weight dextran 500 mL intravenous 4 times a day Participant (baseline) characteristics: age/sex (male, female)/baseline hearing loss at start of therapy/start of initial treatment/start of treatment/baseline SDS:
Inclusion criteria: at least a 30 dB hearing loss occurring over 3 frequencies. The symptoms had to occur over a 72‐hour period. "Poor prognosis" cases defined as meeting at least one of the following criteria: 1) hearing loss > 70 dB HL for 3 subsequent 1‐octave steps in frequency; 2) age of patient > 60 years; 3) the pattern of the audiogram flat or high‐frequency hearing loss; 4) presence of severe vertigo, and 5) time exceeded 2 weeks from onset to initial treatment; non‐ responsive to conventional therapy within the first 7 days Exclusion criteria: 1) a change of ≥ 15 dB in pure tone average (PTA) at 4 frequencies (0.5, 1, 2, 4 kHz) or an increase ≥ 15% in speech discrimination score (SDS) after the first 7 days of conventional steroid therapy; 2) evidence of acute otitis media or chronic otitis media on examination; 3) evidence of retrocochlear disease evident on magnetic resonance imaging; 4) history of otologic surgery; 5) history of Ménière's disease, autoimmune hearing loss, radiation‐induced hearing loss or other potential aetiology for sensorineural hearing loss; 6) history of genetic sensorineural hearing loss or known inner ear anomaly; 7) history of fluctuation of hearing before or after intratympanic therapy |
|
| Interventions |
General comparison: combined versus systemic corticosteroid therapy Intervention group (n = 37*): "combined therapy": intratympanic injections of methylprednisolone 40 mg/mL, 0,5 mL, 4 injections total, 1 x every 2 days + systemic corticosteroid as in comparator group Comparator group (n = 39*): "systemic therapy": 16 mg methylprednisolone oral per day for 1 day and 8 mg per day for another 2 days (continuation of the primary therapy) Use of additional interventions (common to both treatment arms): naftidrofuryl 200 mg oral 3 times a day, diazepam 5 mg oral 3 times a day and low molecular weight heparin 0.4 mL subcutaneous 2 times a day or low molecular weight dextran 500 mL intravenous 4 times a day *modified intention‐to‐treat analysis |
|
| Outcomes |
Primary outcome measure:
Secondary outcomes:
Primary endpoint for hearing threshold evaluation: 60 days Used PTA: 4PTA (0.5, 1, 2, 4 kHz) |
|
| Funding sources | No information available | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Each patient … was given a consecutive number. The odd number patients were classified into a Control group… The even number patients were classified into a TR (transtympanic steroid) group." "Those patients who were classified into the TR group had a chance to choose the treatment protocol." |
| Allocation concealment (selection bias) | High risk | Method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial, no blinding. Patients in the treatment group could refuse therapy after allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement (not mentioned). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No missing outcome data reported but 2 patients refused advised treatment (2.6%). |
| Selective reporting (reporting bias) | High risk | Not all pre‐specified follow‐up time points were reported. |
| Other bias | High risk | No sample size calculation performed. Hearing threshold before treatment in control group not reported. Inclusion criterion "Poor prognosis group" exhibits a possible bias in the selection of study population. Early start of secondary therapy after 7 days only. Treatment delay from onset to secondary treatment in patients not reported but reported treatment delay to initial treatment and duration of initial treatment. |
ATP: adenosine triphosphate CI: confidence interval CMV: cytomegalovirus d: days dB HL: decibels hearing level f: female h: hours HL: hearing loss HSV: herpes simplex virus (I)SSNHL: (idiopathic) sudden sensorineural hearing loss IT: intratympanic m: male MRI: magnetic resonance imaging n.a.: not available NIDCD: National Institute on Deafness and Other Communication Disorders PTA: pure tone average SD: standard deviation SDmax: maximum speech discrimination score SDS: speech discrimination score SE: standard error SPL: sound pressure level SRT: speech reception threshold TM: tympanic membrane WRS: word recognition score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amizadeh 2021 | Double‐blind, parallel‐group randomised controlled trial conducted in Iran between June 2012 and September 2019. It included 51 participants and compared intravenous followed by oral corticosteroid plus intratympanic corticosteroid versus oral corticosteroid alone as primary intervention. It was excluded because the route of administration and dosage of systemic corticosteroid differed between groups. |
| Attanasio 2015 | Non‐blinded, parallel‐group randomised controlled trial conducted in Italy between January 2012 and December 2013. It included 55 participants and compared 2 different protocols of hyperbaric oxygen therapy plus intratympanic corticosteroid treatment as primary intervention. The intervention in both groups included hyperbaric oxygen therapy. This type of comparison was not part of the review. |
| Berjis 2016 | Non‐blinded, parallel‐group randomised controlled trial conducted in Iran between February 2012 and January 2013. It included 50 participants and compared corticosteroid treatment by intratympanic injection of methylprednisolone with intratympanic injection of dexamethasone as secondary intervention. In this study 2 intratympanic treatment protocols using 2 different intratympanically applied drugs were compared. This type of comparison was not part of the review. |
| Chang 2020 | Non‐blinded, parallel‐group randomised controlled trial conducted in China between June 2012 and March 2015. It included 60 participants and compared intratympanic corticosteroid treatment with ear drop corticosteroid treatment as primary intervention. This type of comparison was not part of the review. |
| Chen 2015 | Non‐blinded, parallel‐group randomised controlled trial conducted in China between June and December 2014. It included 68 participants and compared intratympanic corticosteroid treatment with systemic corticosteroid treatment as primary intervention. The comparator group in this study included a mixture of interventions. Most patients were treated with systemic steroids but a subgroup of patients got additional intratympanic steroids as supplementary treatment. |
| Cho 2018 | Non‐blinded, parallel‐group randomised controlled trial conducted in South Korea between July 2014 and September 2016. It included 60 participants and compared combined corticosteroid plus hyperbaric oxygenation treatment with combined corticosteroid treatment alone as primary Intervention. Hyperbaric oxygen therapy was included in the intervention group. This type of comparison was not part of the review. |
| Choo 2017 | Non‐blinded, parallel‐group randomised controlled trial conducted in South Korea between July 2010 and November 2014. It included 117 participants and compared intratympanic corticosteroid treatment, oral corticosteroid treatment and combined corticosteroid treatment as primary intervention separated by low‐ or high‐frequency hearing loss. A comparison of hearing improvement in ISSNHL patients with low‐ and high‐frequency hearing loss was not part of the review. |
| Cvorovic 2013 | Non‐blinded, parallel‐group randomised controlled trial conducted in Serbia between January 2005 and December 2011. It included 155 participants and compared hyperbaric oxygen therapy with intratympanic injection of dexamethasone as secondary intervention. The intervention in the comparator group is hyperbaric oxygen therapy. This type of comparison was not part of the review. |
| Diao 2012 | Non‐blinded, parallel‐group randomised controlled trial with 8 days duration of treatment conducted in China between October 2010 and July 2011. It included 90 participants and combined intratympanic plus systemic corticosteroid treatment with systemic corticosteroid treatment alone as primary intervention. Study population included a high proportion of patients with bilateral sudden hearing loss that raises doubt about whether they represented people with ISSNHL. Further, the unit of analysis in this study was ears instead of patients as is used by this review. |
| Filipo 2013 | Triple‐blinded, parallel‐group randomised controlled trial with 3 days duration of treatment conducted in Italy between August 2011 and March 2012. It included 50 participants and compared intratympanic corticosteroid treatment with intratympanic placebo as primary intervention. It compares the number of patients improved between treatment arms after therapy and hearing improvement as well as frequency‐specific improvement at a 7 days duration of follow‐up. Studies with a follow‐up of 7 days or less were not included in the review. |
| Gui‐li 2018 | Non‐blinded, parallel‐group randomised controlled trial conducted in China between January 2014 and December 2016. It included 60 participants and compared intratympanic corticosteroid treatment accompanied by hyperbaric oxygen therapy with systemic corticosteroid treatment accompanied by hyperbaric oxygen therapy as primary intervention. The interventions in this study include hyperbaric oxygen therapy in the intervention and comparator group. This type of comparison was not part of the review. |
| Han 2021 | Non‐blinded, parallel‐group randomised controlled trial conducted in China between January 2020 and December 2020. It included 176 participants and compared intratympanic injection of corticosteroid versus corticosteroid administered via endoscopic tympanoplasty as primary intervention. This type of comparison was not part of the review. |
| Li 2016 | Non‐blinded, parallel‐group randomised controlled trial conducted in China between January 2006 and January 2014. It included 149 participants and compared intratympanic corticosteroid treatment with intratympanic corticosteroid plus mouse nerve growth factor treatment as primary intervention. This type of comparison was not part of the review. |
| NCT04766853 | This is a trial registration for a single‐blind (participant), parallel‐group randomised controlled trial that compares corticosteroid treatment by intratympanic injection of dexamethasone with intratympanic injection of dexamethasone mixed with hyaluronic acid. This type of comparison was not part of the review. |
| Park 2011 | Non‐blinded, parallel‐group randomised controlled trial conducted in the Republic of Korea between December 2009 and February 2011. It included 92 participants and compared simultaneous systemic and intratympanic corticosteroid treatment with subsequent systemic and intratympanic corticosteroid treatment as primary intervention. In this study 2 protocols of combination therapy (systemic plus intratympanic) were compared. There was no control group for the intratympanic salvage situation without local application. This type of comparison was not part of the review. |
| Rogha 2017 | Non‐blinded, parallel‐group randomised controlled trial conducted in Iran in 2015. It included 40 participants and compared corticosteroid treatment by intratympanic injection of dexamethasone with intratympanic injection of dexamethasone mixed with hyaluronic acid. This type of comparison was not part of the review. |
| Sevil 2016 | Non‐blinded, parallel‐group randomised controlled trial conducted in Turkey between March 2013 and June 2014. It included 88 participants and compared intratympanic corticosteroid treatment accompanied by hyperbaric oxygen therapy with systemic corticosteroid treatment accompanied by hyperbaric oxygen therapy as primary intervention. The interventions in this study include hyperbaric oxygen therapy in the intervention and comparator group. This type of comparison was not part of the review. |
| Song 2018 | Non‐blinded, parallel‐group randomised controlled trial conducted in China between October 2015 and May 2018. It included 48 participants and compared intratympanic corticosteroid treatment with postauricular injection of corticosteroid as primary intervention. This type of comparison was not part of the review. |
| Sun 2016 | Non‐blinded, parallel‐group randomised controlled trial conducted in China between December 2013 and February 2015. It included 90 participants and compared corticosteroid treatment by intratympanic injection of budesonide with intratympanic injection of dexamethasone and a third arm without corticosteroid treatment but a cocktail of different other substances as secondary intervention. Two intratympanic treatment protocols using 2 different intratympanically applied drugs were compared. This type of comparison was not part of the review. |
| Zhou 2006 | Non‐blinded, parallel‐group randomised controlled trial conducted in China between February 2002 and December 2004. It included 50 participants and compared intratympanic corticosteroid treatment accompanied by hyperbaric oxygen therapy with systemic corticosteroid treatment accompanied by hyperbaric oxygen therapy as primary intervention. The interventions include hyperbaric oxygen therapy in the intervention and comparator group. This type of comparison was not part of the review. |
ISSNHL: idiopathic sudden sensorineural hearing loss
Characteristics of ongoing studies [ordered by study ID]
Wang 2021.
| Study name | Clinical study of oral prednisone vs intratympanic injection of dexamethasone as initial treatment for sudden hearing loss |
| Methods | Non‐blinded, parallel‐group randomised controlled trial with 14 days duration of treatment and 1‐month duration of follow‐up |
| Participants |
Inclusion criteria: 1) aged between 18 and 80 years old; 2) unilateral deafness occurred within 72 hours, and the average pure tone hearing threshold of 0.5 KHz, 1 kHz, 2 KHz, 4 kHz was greater than 60 dB; the bilateral hearing was symmetrical in the past; and 3) the time from the time of treatment to the onset of the disease was not more than 14 days Exclusion criteria: 1) previous history of deafness in the affected ear or the opposite ear; 2) acute and chronic otitis media, otosclerosis and other middle ear diseases; 3) congenital or hereditary deafness; 4) abnormal development of the inner ear, such as enlargement of vestibular aqueduct and Mondini malformation; 5) history of ear trauma, ear barotrauma or noise‐induced deafness; 6) inner ear or intracranial tumour; 7) drug‐induced deafness; 8) systemic diseases: tuberculosis, insulin‐dependent diabetes mellitus, rheumatic disease, active atherosclerotic vascular disease, severe mental or psychological disease, history of chemotherapy or other immunosuppressant treatment, pancreatitis, HIV, hepatitis C or hepatitis B infection, chronic renal insufficiency, active herpes zoster infection, severe osteoporosis, head and neck cancer history or radiation therapy; 9) women in pregnancy or lactation |
| Interventions | Group I: intratympanic injection of dexamethasone, 10 mg/mL, 9 times in 14 days Group II: oral prednisolone, 660 mg in total over 14 days |
| Outcomes | Measured before treatment and 1 month after treatment: pure tone threshold, speech recognition rate, VEMP, DHI and THI |
| Starting date | October 2020 |
| Contact information | Study leader: Wang Jing Tel: +86 18917786267 Email: jingwang61@126.com Address: 83 Fenyang Road, Xuhui District, Shanghai, China Postcode: 200031 |
| Notes | ChiCTR, ChiCTR2000036382 Registered on 22 August 2020, prospectively registered |
Yang 2020.
| Study name | A prospective study on the treatment of patients with sudden deafness and diabetes with two different ways of administration |
| Methods | Prospective, non‐blinded, parallel‐group randomised superiority trial |
| Participants | Inclusion criteria: 1) aged 18 to 65 years old; 2) a sudden onset of sensorineural hearing loss within 72 hours, at least in the 3 consecutive frequency of hearing loss of ≥ 30 dB HL; 3) unilateral onset; 4) confirmed diabetes; 5) the time of onset is within 30 days; 6) hearing loss must have been deemed idiopathic |
| Interventions |
Group I (n = 43): 4 x 1 mL doses of 40 mg/mL of methylprednisolone over a 1‐week period, with a dose administered every 2 days via tympanic membrane injection into the middle ear Group II (n = 43): intravenous methylprednisolone (1 mg/kg/day, maximal dose 60 mg/day) for 5 days |
| Outcomes | Primary outcome is the change in hearing threshold from the first audiogram to the 30‐day follow‐up audiogram Secondary outcome measures will include pure tone average (PTA) at 90‐day follow‐up, visual analogue tinnitus scale, visual analogue vertigo scale, visual analogue aural fullness scale, fasting blood glucose and 2‐hour postprandial blood glucose during treatment and the change in glycosylated haemoglobin (HbA1C) levels. Vital signs and otological physical examination will be performed at each follow‐up visit. |
| Starting date | Planned study execute time: 1 January 2018 to 31 December 2022 |
| Contact information | Weiqiang Yang, Peking Unversity Shenzhen hospital, 1120 Lianhua Road, Futian District, Shenzhen, Guangdong, China, e‐mail: 497450210@qq.com |
| Notes | ChiCTR, ChiCTR1800015954 Registered on 2 May 2018, retrospectively registered |
DHI: Dizziness Handicap Inventory; THI: Tinnitus Handicap Inventory; VEMP: vestibular evoked myogenic potential
Differences between protocol and review
We have supplemented the methods section with a detailed description of the selection and data extraction process that we used during the work on the review, i.e. detailed inclusion criteria for studies, inclusion of publications written in the Chinese language, selection of treatment arms for inclusion from included studies, numbers of people included in selection and data extraction process, and documented study parameters that were crucial for the review.
Since the publication of the protocol it has been shown in a meta‐analysis on studies using intratympanically applied corticosteroids that the change of hearing threshold depends on the hearing threshold before the intervention and also on the start of therapy (Liebau 2017). The studies compared in this review, however, differed with respect to these baseline parameters. In addition, the clinical relevance of the outcomes "mean change of hearing threshold" and "percentage of patients having improved hearing" also depends the initial hearing loss. For patients, it is most likely more relevant what final hearing level they will reach, and especially whether the intervention will lead to "serviceable hearing". Since the information on how many patients reached "serviceable hearing" is missing in nearly all studies selected in this review, we analysed the mean "final hearing threshold" as a secondary outcome parameter.
During the review work, we identified a comparison that had not been included in the protocol but is important to report. Therefore, we added the following comparison to the analysis: intratympanic versus intratympanic plus systemic corticosteroids (combined therapy). For completeness of the possible comparisons, we have also added "intratympanic corticosteroids plus systemic corticosteroids (combined therapy) versus no treatment or versus placebo", however, no studies were identified for this comparison for either primary or secondary therapy of ISSNHL.
Due to the lack of availability of individual patient data and the limited number of studies per type of comparison, we could not perform the following intended subgroup analyses: 1) degree of hearing loss at initial presentation; 2) age of patients; 3) time before start of intratympanic treatment; 4) duration of intratympanic treatment; 5) dose of intratympanic treatment; 6) presence of vertigo and/or tinnitus.
The simply stated objective in the abstract replaced the text in the main 'Objectives' section, in order to make them identical.
In the manuscript title the word 'glucocorticoids' has been replaced by 'corticosteroids', since this term is more often used and generally accepted in the literature on ISSNHL.
Contributions of authors
Stefan K Plontke: scoped and co‐ordinated the review, designed and wrote the protocol; clinical guidance at all stages of the review; screened the search results and selected studies; carried out data extraction; risk of bias assessment; reviewed the analyses; wrote the abstract and the plain language summary of the review; wrote, reviewed and edited the text of the review.
Christoph Meisner: designed and wrote the protocol; screened the search results and selected studies; carried out data extraction; risk of bias assessment and statistical analyses; reviewed the analyses; wrote, reviewed and edited the text of the review.
Arne Liebau: screened the search results and selected studies; carried out data extraction; risk of bias assessment; reviewed the analyses; wrote, reviewed and edited the text of the review.
Stefan K Plontke, Christoph Meisner and Arne Liebau contributed equally to this review.
The following authors are listed in alphabetical order:
Sumit Agrawal: clinical guidance at all stages of the review; screened the search results and selected studies; carried out data extraction; reviewed the analyses; reviewed and edited the text of the review.
Per Caye‐Thomasen: clinical guidance at all stages of the review; screened the search results and selected studies; carried out data extraction; reviewed the analyses; reviewed and edited the text of the review.
Kevin Galbraith: screened the search results and selected studies; carried out data extraction; reviewed the analyses; reviewed and edited the text of the review.
Anthony A Mikulec: clinical guidance at all stages of the review; screened the search results and selected studies; carried out data extraction; reviewed the analyses; reviewed and edited the text of the review.
Lorne Parnes: clinical guidance at all stages of the review; screened the search results and selected studies; carried out data extraction; reviewed the analyses; reviewed and edited the text of the review.
Yaamini Premakumar: screened the search results and selected studies; carried out data extraction; reviewed the analyses; reviewed the text of the review.
Julia Reiber: carried out data extraction; risk of bias assessment; wrote, reviewed and edited the text of the review.
Anne GM Schilder: clinical guidance at the later stages of the review; reviewed the analyses; reviewed and edited the text of the review.
Sources of support
Internal sources
-
State of Saxony‐Anhalt and Martin Luther University Halle‐Wittenberg, Germany
The conduct of this review was supported by the state of Saxony‐Anhalt through the Martin Luther University Halle‐Wittenberg by means of the basic research funding for universities to SKP and AL.
External sources
-
National Institute for Health Research, UK
Infrastructure funding for Cochrane ENT
-
Federal Ministry of Education and Research ‐ BMBF, Germany
This project was supported by the Federal Ministry of Education and Research ‐ BMBF, grand no. 01KG2019 "ITKORT ‐ Intratympanic glucocorticosteroids for the therapy of idiopathic sudden sensorineural hearing loss" to SKP
Declarations of interest
Stefan K Plontke served as study co‐ordinator and the second author (CM) as the responsible biostatistician of a randomised controlled trial within the focus of this review (Plontke 2009). The sponsor of this completed trial was the University of Tübingen Medical School (academic sponsor, non‐industrial). These authors were not involved in data extraction or risk of bias assessment for this study.
Stefan K Plontke also serves as co‐ordinating investigator and the second author (CM) as the responsible biostatistician of a randomised controlled trial on the efficacy and safety of systemic high‐dose glucocorticoid treatment for idiopathic sudden sensorineural hearing loss in adults (HODOKORT) (EudraCT Nr. 2015‐002602‐36).
Stefan K Plontke: AudioCure PharmaGmbH, Berlin, Germany; Astellas Pharma Inc., Tokyo (no payment), Japan: consultant; MED‐EL Austria and MED‐EL Germany: travel reimbursement for speaking engagement; MED‐EL Austria and MED‐EL Germany, OticonMedical, Denmark; Cochlear Ltd., Australia; Federal Ministry of Education and Research in Germany (Bundesministeriumfür Bildung und Forschung): research projects; Society of ENT physicians in Germany (BV‐HNO e.V); Merck Serono, Darmstadt; Infectopharm, Heppenheim; Dr. Willmar Schwabe GmbH&Co. KG, Kralsruhe, Germany: lecture fees and travel reimbursement. The above industry relations were not related to any treatment in this review. The above industry relations did not sponsor any part of this work.
Christoph Meisner: AudioCure PharmaGmbH, Berlin, Germany: consultant; palleos healthcare GmbH, Wiesbaden, Germany; WSG ‐ Westdeutsche Studiengruppe GmbH, Mönchengladbach, Germany: Member of DSMB. The above industry relations were not related to any treatment in this review. The above industry relations did not sponsor any part of this work.
Arne Liebau: MED‐EL Austria and MED‐EL Germany; Dr. Willmar Schwabe GmbH&Co. KG, Karlsruhe, Germany (research projects).
Sumit Agrawal: none known.
Per Caye‐Thomasen: none known.
Kevin Galbraith: none known.
Anthony A Mikulec: Otonomy Inc., San Diego, USA: clinical trial site principal investigator, clinical trial safety review committee member, ad hoc consultant.
Lorne Parnes: none known.
Yaamini Premakumar: none known.
Julia Reiber: none known.
Anne GM Schilder: Professor Schilder's research at University College London is funded by the NIHR and EU Horizon2020. Anne Schilder is the national chair of the NIHR Clinical Research Network ENT Specialty and Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Trials Initiative. As director of the NIHR UCLH Biomedical Research Centre Hearing Theme, she advises companies developing innovative hearing treatments on clinical trial design and delivery.
Stefan K. Plontke, Christoph Meisner and Arne Liebau contributed equally to this study.
Stefan K. Plontke, Christoph Meisner and Arne Liebau contributed equally to this study.
Stefan K. Plontke, Christoph Meisner and Arne Liebau contributed equally to this study.
New
References
References to studies included in this review
Ahn 2008 {published data only}
- Ahn JH, Yoo MH, Yoon TH, Chung JW. Can intratympanic dexamethasone added to systemic steroids improve hearing outcome in patients with sudden deafness? Laryngoscope 2008;118(2):279-82. [DOI] [PubMed] [Google Scholar]
Al‐Shehri 2015 {published data only}
- Al-Shehri AMS. Intratympanic vs. oral steroids for treatment of idiopathic sudden sensorineural hearing loss: a randomized controlled study. British Journal of Medicine & Medical Research 2015;11(6):1-6. [Google Scholar]
Arastou 2013 {published data only}
- Arastou S, Tajedini A, Borghei P. Combined intratympanic and systemic steroid therapy for poor-prognosis sudden sensorineural hearing loss. Iranian Journal of Otorhinolaryngology 2013;25(70):23-8. [PMC free article] [PubMed] [Google Scholar]
Arslan 2011 {published data only}
- Arslan N, Oğuz H, Demirci M, Şafak MA, İslam A, Kaytez SK, et al. Combined intratympanic and systemic use of steroids for idiopathic sudden sensorineural hearing loss. Otology & Neurotology 2011;32(3):393-7. [DOI] [PubMed] [Google Scholar]
Ashtiani 2018 {published data only}
- Ashtiani MK, Firouzi F, Bastaninejad S, Dabiri S, Nasirmohtaram S, Saeedi N, et al. Efficacy of systemic and intratympanic corticosteroid combination therapy versus intratympanic or systemic therapy in patients with idiopathic sudden sensorineural hearing loss: a randomized controlled trial. Archives of Oto-rhino-laryngology 2018;275(1):89-97. [DOI] [PubMed] [Google Scholar]
Battaglia 2008 {published data only}
- Battaglia A, Burchette R, Cueva R. Combination therapy (intratympanic dexamethasone + high-dose prednisone taper) for the treatment of idiopathic sudden sensorineural hearing loss. Otology & Neurotology 2008;29(4):453-60. [DOI] [PubMed] [Google Scholar]
Chang 2010 {published data only}
- Chang WX, Zhong HL, Cai X, Gan Q. Dexamethasone injection in the middle ear for high altitude refractory sudden deafness. Sichuan Medical Journal 2010;31(1):71. [Google Scholar]
Choi 2011 {published data only}
- Choi SJ, Lee YH, Kim YH. Comparison of the efficacy of systemic and combined highly frequent intratympanic steroid treatment on sudden sensorineural hearing loss. Korean Journal of Audiology 2011;15:133-6. [Google Scholar]
Dispenza 2011 {published data only}
- Dispenza F, Amodio E, De Stefano A, Gallina S, Marchese D, Mathur N, et al. Treatment of sudden sensorineural hearing loss with transtympanic injection of steroids as single therapy: a randomized clinical study. European Archives of Oto-rhino-laryngology 2011;268(9):1273-8. [DOI] [PubMed] [Google Scholar]
Ermutlu 2017 {published data only}
- Ermutlu G, Süslü N, Yılmaz T, Saraç S. Sudden hearing loss: an effectivity comparison of intratympanic and systemic steroid treatments. European Archives of Oto-rhino-laryngology 2017;274(10):3585-91. [DOI] [PubMed] [Google Scholar]
Gundogan 2013 {published data only}
- Gundogan O, Pinar E, Imre A, Ozturkcan S, Cokmez O, Yigiter AC. Therapeutic efficacy of the combination of intratympanic methylprednisolone and oral steroid for idiopathic sudden deafness. Otolaryngology - Head & Neck Surgery 2013;149:753-8. [DOI] [PubMed] [Google Scholar]
Ho 2004 {published data only}
- Ho HG, Lin HC, Shu MT, Yang CC, Tsai HT. Effectiveness of intratympanic dexamethasone injection in sudden-deafness patients as salvage treatment. Laryngoscope 2004;114(7):1184-9. [DOI] [PubMed] [Google Scholar]
Hong 2009 {published data only}
- Hong SM, Park CH, Lee JH. Hearing outcomes of daily intratympanic dexamethasone alone as a primary treatment modality for ISSHL. Otolaryngology - Head and Neck Surgery 2009;141(5):579-83. [DOI] [PubMed] [Google Scholar]
Huang 2021 {published data only}
- Huang J, Yang L, Cao X, Wang W. Differences in hearing recovery following intratympanic alone or intravenous dexamethasone with rescue intratympanic steroids for sudden sensorineural hearing loss: a randomised trial. Clinical Otolaryngology 2021;46(3):546-51. [DOI] [PubMed] [Google Scholar]
Koltsidopoulos 2013 {published data only}
- Koltsidopoulos P, Bibas A, Sismanis A, Tzonou A, Seggas I. Intratympanic and systemic steroids for sudden hearing loss. Otology & Neurotology 2013;34:771-6. [DOI] [PubMed] [Google Scholar]
Kosyakov 2011 {published data only}
- Kosyakov S, Atanesyan A, Gunenkov A, Ashkhatunyan E, Kurlova A. Intratympanic steroids for sudden sensorineural hearing loss. Journal of International Advanced Otology 2011;7(3):323-32. [Google Scholar]
Lee 2011 {published data only}
- Lee JB, Choi SJ, Park K, Park HY, Choo OS, Choung YH. The efficiency of intratympanic dexamethasone injection as a sequential treatment after initial systemic steroid therapy for sudden sensorineural hearing loss. European Archives of Oto-rhino-laryngology 2011;268(6):833-9. [DOI] [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li P, Zeng XL, Ye J, Yang QT, Zhang GH, Li Y. Intratympanic methylprednisolone improves hearing function in refractory sudden sensorineural hearing loss: a control study. Audiology & Neurotology 2011;16(3):198-202. [DOI] [PubMed] [Google Scholar]
Lim 2013 {published data only}
- Lim HJ, Kim YT, Choi SJ, Lee JB, Park HY, Park K, et al. Efficacy of 3 different steroid treatments for sudden sensorineural hearing loss: a prospective, randomized trial. Otolaryngology - Head & Neck Surgery 2013;148(1):121-7. [DOI] [PubMed] [Google Scholar]
- Lim HJ, Kim YT, Park HY, Park K, Choung YH, Choi SJ, et al. Response to: "Possible errors in hearing recovery results" from Kai-Min Fang. Otolaryngology - Head & Neck Surgery 2013;148(6):1063. [DOI: 10.1177/0194599813486090] [DOI] [PubMed] [Google Scholar]
Peng 2008 {published data only}
- Peng Y, Xiong S, Cheng Y, Qi YF, Yang Y. Clinical investigation of different routes of administration of dexamethasone on sudden deafness [Lin chuang er bi yan hou tou jing wai ke za zhi]. Journal of Clinical Otorhinolaryngology, Head and Neck Surgery (Chinese) 2008;22(10):442-5. [PubMed] [Google Scholar]
Plontke 2009 {published data only}
- NCT00335920. Safety and efficacy of intratympanic application of dexamethasone via catheter in patients with sudden hearing loss. https://clinicaltrials.gov/ct2/show/NCT00335920 (first received 12 June 2006).
- Plontke SK, Lowenheim H, Mertens J, Engel C, Meisner C, Weidner A, et al. Randomized, double blind, placebo controlled trial on the safety and efficacy of continuous intratympanic dexamethasone delivered via a round window catheter for severe to profound sudden idiopathic sensorineural hearing loss after failure of systemic therapy. Laryngoscope 2009;119(2):359-69. [DOI] [PubMed] [Google Scholar]
Qu 2015 {published data only}
- Qu Y, Chen H, Zhang H, Guo M. Analysis the treatment of sudden sensorineural hearing loss with steroid from different administration routes [Lin chuang er bi yan hou tou jing wai ke za zhi]. Journal of Clinical Otorhinolaryngology, Head and Neck Surgery [Chinese] 2015;29(4):324-6. [PubMed] [Google Scholar]
Rauch 2011 {published data only}
- Rauch SD, Halpin CF, Antonelli PJ, Babu S, Carey JP, Gantz BJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA 2011;305(20):2071-9. [DOI] [PubMed] [Google Scholar]
Rupasinghe 2017 {published data only}
- Rupasinghe RAST. Comparison - Effectiveness of oral steroid versus intratympanic dexamethasone for sudden onset sensorineural hearing loss. Ceylon Journal of Otolaryngology 2017;5(1):7-13. [Google Scholar]
Swachia 2016 {published data only}
- Swachia K, Sharma D, Singh J. Efficacy of oral vs. intratympanic corticosteroids in sudden sensorineural hearing loss. Journal of Basic and Clinical Physiology and Pharmacology 2016;27(4):371-7. [DOI] [PubMed] [Google Scholar]
Tong 2021 {published data only}
- Duan M. Data on frequency specific hearing changes (table 5) [personal communication]. Email to K Webster 9 October 2021.
- Tong B, Wang Q, Dai Q, Hellstrom S, Duan M. Efficacy of various corticosteroid treatment modalities for the initial treatment of idiopathic sudden hearing loss: a prospective randomized controlled trial. Audiology & Neurotology 2021;26(1):45-52. [DOI] [PubMed] [Google Scholar]
Tsounis 2018 {published data only}
- Tsounis M, Psillas G, Tsalighopoulos M, Vital V, Maroudias N, Markou K. Systemic, intratympanic and combined administration of steroids for sudden hearing loss. A prospective randomized multicenter trial. Otorhinolaryngology 2018;275(1):103-10. [DOI] [PubMed] [Google Scholar]
Wu 2011 {published data only}
- Wu HP, Chou YF, Yu SH, Wang CP, Hsu CJ, Chen PR. Intratympanic steroid injections as a salvage treatment for sudden sensorineural hearing loss: a randomized, double-blind, placebo-controlled study. Otology & Neurotology 2011;32(5):774-9. [DOI] [PubMed] [Google Scholar]
Xenellis 2006 {published data only}
- Xenellis J, Papadimitriou N, Nikolopoulos T, Maragoudakis P, Segas J, Tzagaroulakis A, et al. Intratympanic steroid treatment in idiopathic sudden sensorineural hearing loss: a control study. Otolaryngology - Head & Neck Surgery 2006;134(6):940-5. [DOI] [PubMed] [Google Scholar]
Zhou 2011 {published data only}
- Zhou Y, Zheng H, Zhang Q, Campione PA. Early transtympanic steroid injection in patients with 'poor prognosis' idiopathic sensorineural sudden hearing loss. ORL; Journal for Oto-rhino-laryngology and Its Related Specialties 2011;73(1):31-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amizadeh 2021 {published data only}
- Amizadeh M, Mozafarnia K, Moslemikia J, Naghibzadeh-Tahami A. Combination of pulse steroid with intratympanic injections in sudden sensorineural hearing loss. Iranian Journal of Otorhinolaryngology 2021;33(1):9-13. [CENTRAL: CN-02287535] [EMBASE: 2012412644] [DOI] [PMC free article] [PubMed] [Google Scholar]
Attanasio 2015 {published data only}
- Attanasio G, Covelli E, Cagnoni L, Masci E, Ferraro D, Mancini P, et al. Does the addition of a second daily session of hyperbaric oxygen therapy to intratympanic steroid influence the outcomes of sudden hearing loss? Acta Otorhinolaryngologica Italica 2015;35(4):272-6. [PMC free article] [PubMed] [Google Scholar]
Berjis 2016 {published data only}
- Berjis N, Soheilipour S, Musavi A, Hashemi SM. Intratympanic dexamethasone injection vs methylprednisolone for the treatment of refractory sudden sensorineural hearing loss. Advanced Biomedical Research 2016;5:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2020 {published data only}
- Chang WT, Zee B, Lee HSH, Tong MCF. Dexamethasone eardrop with grommet placement vs intratympanic steroid injection for sudden sensorineural hearing loss: a randomized prospective clinical trial. American Journal of Otolaryngology 2020;41(4):102515. [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen J, Yang J, Jia H, Shi J, Li Y, Wu H. Efficacy of intratympanic steroid injection as supplementary or initial treatment for sudden sensorineural hearing loss. Lin chuang er bi yan hou tou jing wai ke za zhi [Journal of Clinical Otorhinolaryngology, Head and Neck Surgery] 2015;29(19):1691-4. [PubMed] [Google Scholar]
Cho 2018 {published data only}
- Cho I, Lee HM, Choi SW, Kong SK, Lee IW, Goh EK, et al. Comparison of two different treatment protocols using systemic and intratympanic steroids with and without hyperbaric oxygen therapy in patients with severe to profound idiopathic sudden sensorineural hearing loss: a randomized controlled trial. Audiology & Neuro-otology 2018;23(4):199-207. [DOI] [PubMed] [Google Scholar]
Choo 2017 {published data only}
- Choo OS, Yang SM, Park HY, Lee JB, Jang JH, Choi SJ, et al. Differences in clinical characteristics and prognosis of sudden low- and high-frequency hearing loss. Laryngoscope 2017;127(8):1878-84. [DOI] [PubMed] [Google Scholar]
Cvorovic 2013 {published data only}
- Cvorovic L, Jovanovic MB, Milutinovic Z, Arsovic N, Djeric D. Randomized prospective trial of hyperbaric oxygen therapy and intratympanic steroid injection as salvage treatment of sudden sensorineural hearing loss. Otology & Neurotology 2013;34(6):1021-6. [DOI] [PubMed] [Google Scholar]
Diao 2012 {published data only}
- Diao Y, Dong M. The observation on curative effect of 90 cases of sudden hearing loss. Journal of Clinical Otorhinolaryngology, Head and Neck Surgery [Chinese] 2012;26(7):306-8. [PubMed] [Google Scholar]
Filipo 2013 {published data only}
- Filipo R, Attanasio G, Russo FY, Viccaro M, Mancini P, Covelli E. Intratympanic steroid therapy in moderate sudden hearing loss: a randomized, triple-blind, placebo-controlled trial. Laryngoscope 2013;123(3):774-8. [DOI] [PubMed] [Google Scholar]
Gui‐li 2018 {published data only}
- Xu G, Lin D, Lin Q. Clinical observations of glucocorticoid combined with hyperbaric oxygen on patients with sudden sensorineural hearing loss. Chinese Scientific Journal of Hearing and Speech Rehabilitation 2018;16(1):22-5. [Google Scholar]
Han 2021 {published data only}
- Han X, Wang B, Li C, Wang X, Zhang H, Yang Q, et al. Observation on the efficacy of Eustachian tube dilation hormone under the guidance of endoscope in the treatment of sudden deafness. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke za Zhi [Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery] 2021;35(5):424-7. [CENTRAL: CN-02296088] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Zheng LI, Chen YAO, Xiaohang CAI. To study the effect of intratympanic injection with mouse nerve growth factor and methylprednisolone on sudden deafness. Lin Chuang er bi yan hou tou Jing wai ke za zhi [Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery] 2016;30(21):1728-31. [DOI] [PubMed] [Google Scholar]
NCT04766853 {published data only}
- NCT04766853. Verification of the efficacy/safety of the dual drug delivery for hearing loss [Verification of the efficacy / safety of the dual drug injectable delivery vehicle for treating intractable hearing loss (pilot study)]. https://clinicaltrials.gov/show/NCT04766853 (first received 23 February 2021). [CENTRAL: CN-02249206]
Park 2011 {published data only}
- Park MK, Lee CK, Park KH, Lee JD, Lee CG, Lee BD. Simultaneous versus subsequent intratympanic dexamethasone for idiopathic sudden sensorineural hearing loss. Otolaryngology - Head & Neck Surgery 2011;145(6):1016-21. [DOI] [PubMed] [Google Scholar]
Rogha 2017 {published data only}
- Rogha M, Kalkoo A. Therapeutic effect of intra-tympanic dexamethasone-hyaluronic acid combination in sudden sensorineural hearing loss. Iran Journal of Otorhinolaryngology 2017;29(94):255-60. [PMC free article] [PubMed] [Google Scholar]
Sevil 2016 {published data only}
- Sevil E, Bercin S, Muderris T, Gul F, Kiris M. Comparison of two different steroid treatments with hyperbaric oxygen for idiopathic sudden sensorineural hearing loss. European Archives of Oto-rhino-laryngology 2016;273(9):2419-26. [DOI] [PubMed] [Google Scholar]
Song 2018 {published data only}
- Song J, Zhang L, Chen Y. Analysis of the treatment effects of refractory sudden total frequency deafness with steroid from different topical administration routes. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2018;22(24):1897-9. [DOI] [PubMed] [Google Scholar]
Sun 2016 {published data only}
- Sun RX. Intratympatic budesonide injection for treatment of refractory sudden sensorineural hearing loss. Lin chuang er bi yan hou tou jing wai ke za zhi [Journal of Clinical Otorhinolaryngology, Head and Neck Surgery] 2016;30(12):972-7. [DOI] [PubMed] [Google Scholar]
Zhou 2006 {published data only}
- Zhou XY, Tao Q, Lv LY. The treatment for sudden deafness with intratympanic dexamethasone injection. Chinese Journal of Otorhinolaryngology - Skull Base Surgery 2006;12(1):47-8. [Google Scholar]
References to ongoing studies
Wang 2021 {published data only}
- ChiCTR2000036382. Clinical study of oral prednisone vs intratympanic injection of dexamethasone as initial treatment for sudden hearing loss. http://www.chictr.org.cn/hvshowproject.aspx?id=53824 (first received 22 August 2020).
Yang 2020 {published data only}
- ChiCTR1800015954. A prospective study on the treatment of patients with sudden deafness and diabetes with two different ways of administration. http://www.chictr.org.cn/showproj.aspx?proj=25326 (first received 2 May 2018).
Additional references
Agarwal 2009
- Agarwal L, Pothier DD. Vasodilators and vasoactive substances for idiopathic sudden sensorineural hearing loss. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD003422. [DOI: 10.1002/14651858.CD003422.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmadzai 2019
- Ahmadzai N, Kilty S, Cheng W, Esmaeilisaraji L, Wolfe D, Bonaparte JP, et al. A systematic review and network metaanalysis of existing pharmacologic therapies in patients with idiopathic sudden sensorineural hearing loss. PLOS One 2019;14(9):e0221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akeroyd 2015
- Akeroyd MA, Arlinger S, Bentler RA, Boothroyd A, Dillier N, Dreschler WA, et al. International Collegium of Rehabilitative Audiology (ICRA) recommendations for the construction of multilingual speech tests. ICRA Working Group on Multilingual Speech Tests. International Journal of Audiology 2015;54 Suppl 2:17-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ashtiani 2012
- Khorsandi Ashtiani MT, Borgheie P, Yazdani N, Maghsoud S. The effect of intratympanic dexamethasone with oral prednisolone as a primary treatment in idiopathic sudden sensorineural hearing loss. Iranian Journal of Otorhinolaryngology 2012;24(66):19-22. [PMC free article] [PubMed] [Google Scholar]
Bachmann 2001
- Bachmann G, Su J, Zumegen C, Wittekindt C, Michel O. Permeability of the round window membrane for prednisolone-21-hydrogen succinate. Prednisolone content of the perilymph after local administration vs. systemic injection [German]. HNO 2001;49(7):538-42. [DOI] [PubMed] [Google Scholar]
Battaglia 2014
- Battaglia A, Lualhati A, Lin H, Burchette R, Cueva R. A prospective, multi-centered study of the treatment of idiopathic sudden sensorineural hearing loss with combination therapy versus high-dose prednisone alone: a 139 patient follow-up. Otology & Neurotology 2014;35(6):1091-8. [DOI] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637-9. [DOI] [PubMed] [Google Scholar]
Bennett 2007
- Bennett MH, Kertesz T, Yeung P. Hyperbaric oxygen for idiopathic sudden sensorineural hearing loss and tinnitus. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD004739. [DOI: 10.1002/14651858.CD004739.pub3] [DOI] [PubMed] [Google Scholar]
Bennett 2012
- Bennett MH, Kertesz T, Perleth M, Yeung P, Lehm JP. Hyperbaric oxygen for idiopathic sudden sensorineural hearing loss and tinnitus. Cochrane Database of Systematic Reviews 2012, Issue 10. Art. No: CD004739. [DOI: 10.1002/14651858.CD004739.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bird 2007
- Bird PA, Begg EJ, Zhang M, Keast AT, Murray DP, Balkany TJ. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otology & Neurotology 2007;28(8):1124-30. [DOI] [PubMed] [Google Scholar]
Bird 2011
- Bird PA, Murray DP, Zhang M, Begg EJ. Intratympanic versus intravenous delivery of dexamethasone and dexamethasone sodium phosphate to cochlear perilymph. Otology & Neurotology 2011;32(6):933-6. [DOI] [PubMed] [Google Scholar]
Byl 1977
- Byl FM. Seventy-six cases of presumed sudden hearing loss occurring in 1973: prognosis and incidence. Laryngoscope 1977;87(5 Pt 1):817-25. [DOI] [PubMed] [Google Scholar]
Carlsson 2011
- Carlsson PI, Hall M, Lind KJ, Danermark B. Quality of life, psychosocial consequences, and audiological rehabilitation after sudden sensorineural hearing loss. International Journal of Audiology 2011;50(2):139-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chandrasekhar 2000
- Chandrasekhar SS, Rubinstein RY, Kwartler JA, Gatz M, Connelly PE, Huang E, et al. Dexamethasone pharmacokinetics in the inner ear: comparison of route of administration and use of facilitating agents. Otolaryngology - Head and Neck Surgery 2000;122(4):521-8. [DOI] [PubMed] [Google Scholar]
Chandrasekhar 2019
- Chandrasekhar SS, Tsai Do BS, Schwartz SR, Bontempo LJ, Faucett EA, Finestone SA, et al. Clinical practice guideline: sudden hearing loss (Update). Otolaryngology - Head and Neck Surgery 2019;161(1 Suppl):S1-S45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2003
- Chen CY, Halpin C, Rauch SD. Oral steroid treatment of sudden sensorineural hearing loss: a ten year retrospective analysis. Otology & Neurotology 2003;24(5):728-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Conlin 2007a
- Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: I. A systematic review. Archives of Otolaryngology - Head and Neck Surgery 2007a;133(6):573-81. [DOI] [PubMed] [Google Scholar]
Conlin 2007b
- Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. A meta-analysis. Archives of Otolaryngology - Head and Neck Surgery 2007;133(6):582-6. [DOI] [PubMed] [Google Scholar]
Crane 2015
- Crane RA, Camilon M, Nguyen S, Meyer TA. Steroids for treatment of sudden sensorineural hearing loss: a meta-analysis of randomized controlled trials. Laryngoscope 2015;125(1):209-17. [DOI] [PubMed] [Google Scholar]
Desloovere 1988
- Desloovere C, Meyer-Breiting E, Ilberg C. Randomized double-blind study of therapy of sudden deafness: initial results [Randomisierte Doppelblindstudie zur Hörsturztherapie: Erste Ergebnisse]. HNO 1988;36(10):417-22. [PubMed] [Google Scholar]
Eftekharian 2016
- Eftekharian A, Amizadeh M. Pulse steroid therapy in idiopathic sudden sensorineural hearing loss: a randomized controlled clinical trial. Laryngoscope 2016;126(1):150-5. [DOI] [PubMed] [Google Scholar]
El Kechai 2015
- El Kechai N, Agnely F, Mamelle E, Nguyen Y, Ferrary E, Bochot A. Recent advances in local drug delivery to the inner ear. International journal of pharmaceutics 2015;494(1):83-101. [PMID: ] [DOI] [PubMed] [Google Scholar]
El Sabbagh 2016
- El Sabbagh NG, Sewitch MJ, Bezdjian A, Daniel SJ. Intratympanic dexamethasone in sudden sensorineural hearing loss: a systematic review and meta-analysis. Laryngoscope 2017;127(8):1897–908. [DOI] [PubMed] [Google Scholar]
Eshraghi 2006
- Eshraghi AA, Van de Water TR. Cochlear implantation trauma and noise-induced hearing loss: apoptosis and therapeutic strategies. The Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary biology 2006;288(4):473-81. [DOI] [PubMed] [Google Scholar]
Gao 2016
- Gao Y, Liu D. Combined intratympanic and systemic use of steroids for idiopathic sudden sensorineural hearing loss: a meta-analysis. European Archives of Oto-rhino-laryngology 2016;273(11):3699-711. [DOI] [PubMed] [Google Scholar]
Garavello 2012
- Garavello W, Galluzzi F, Gaini RM, Zanetti D. Intratympanic steroid treatment for sudden deafness: a meta-analysis of randomized controlled trials. Otology & Neurotology 2012;33(5):724-9. [DOI] [PubMed] [Google Scholar]
Garcia‐Berrocal 2008
- Garcia-Berrocal JR, Ramirez-Camacho R, Lobo D, Trinidad A, Verdaguer JM. Adverse effects of glucocorticoid therapy for inner ear disorders. ORL; Journal for Oto-rhino-laryngology and Its Related Specialties 2008;70(4):271-4. [DOI] [PubMed] [Google Scholar]
Grossmann 2004
- Grossmann C, Scholz T, Rochel M, Bumke-Vogt C, Oelkers W, Pfeiffer AF, et al. Transactivation via the human glucocorticoid and mineralocorticoid receptor by therapeutically used steroids in CV-1 cells: a comparison of their glucocorticoid and mineralocorticoid properties. European Journal of Endocrinology 2004;151(3):397-406. [DOI] [PubMed] [Google Scholar]
Gul 2016
- Gul F, Muderris T, Yalciner G, Sevil E, Bercin S, Ergin M, et al. A comprehensive study of oxidative stress in sudden hearing loss. European Archives of Oto-rhino-laryngology 2016;274(3):1301-8. [DOI] [PubMed] [Google Scholar]
Günel 2015
- Günel C, Başal Y, Toka A, Eryılmaz A, Kurt Ömürlü I. Efficacy of low-dose intratympanic dexamethasone for sudden hearing loss. Auris Nasus Larynx 2015;42(4):284-7. [DOI] [PubMed] [Google Scholar]
Gurgel 2012
- Gurgel RK, Jackler RK, Dobie RA, Popelka GR. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngology--Head and Neck Surgery 2012;147(5):803-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Haake 2009
- Haake SM, Dinh CT, Chen S, Eshraghi AA, Van De Water TR. Dexamethasone protects auditory hair cells against TNFalpha-initiated apoptosis via activation of PI3K/Akt and NFkappaB signaling. Hearing Research 2009;255(1-2):22-32. [DOI] [PubMed] [Google Scholar]
Han 2017
- Han X, Yin X, Du X, Sun C. Combined intratympanic and systemic use of steroids as a first-line treatment for sudden sensorineural hearing loss: a meta-analysis of randomized, controlled trials. Otology & Neurotology 2017;38(4):487-95. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 Available from www.cochrane-handbook.org.
Handbook 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Haynes 2007
- Haynes DS, O'Malley M, Cohen S, Watford K, Labadie RF. Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. Laryngoscope 2007;117(1):3-15. [DOI] [PubMed] [Google Scholar]
Hoang 2009
- Hoang KN, Dinh CT, Bas E, Chen S, Eshraghi AA, Van De Water TR. Dexamethasone treatment of naive organ of Corti explants alters the expression pattern of apoptosis-related genes. Brain Research 2009;1301:1-8. [DOI] [PubMed] [Google Scholar]
Hoskison 2013
- Hoskison E, Daniel M, Al-Zahid S, Shakesheff KM, Bayston R, Birchall JP. Drug delivery to the ear. Therapeutic Delivery 2013;4(1):115-24. [DOI] [PubMed] [Google Scholar]
Hughes 1996
- Hughes GB, Freedman MA, Haberkamp TJ, Guay ME. Sudden sensorineural hearing loss. Otolaryngologic Clinics of North America 1996;29(3):393-405. [PubMed] [Google Scholar]
Hultcrantz 2014
- Hultcrantz E, Nosrati-Zarenoe R. Corticosteroid treatment of idiopathic sudden sensorineural hearing loss: analysis of an RCT and material drawn from the Swedish national database. European Archives of Oto-rhino-laryngology 2015;272(11):3169-75. [DOI] [PubMed] [Google Scholar]
Jenkinson 1997
- Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? Journal of Public Health Medicine 1997;19(2):179-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Katiri 2020
- Katiri R, Hall DA, Buggy N, Hogan N, Horobin A, de Heyning P, et al. Core Rehabilitation Outcome Set for Single Sided Deafness (CROSSSD) study: protocol for an international consensus on outcome measures for single sided deafness interventions using a modified Delphi survey. Trials 2020;21(1):238. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiliç 2007
- Kiliç R, Safak MA, Oğuz H, Kargin S, Demirci M, Samim E, et al. Intratympanic methylprednisolone for sudden sensorineural hearing loss. Otology & Neurotology 2007;28(3):312-6. [DOI] [PubMed] [Google Scholar]
King 2011
- King EB, Salt AN, Eastwood HT, O'Leary SJ. Direct entry of gadolinium into the vestibule following intratympanic applications in Guinea pigs and the influence of cochlear implantation. Journal of the Association for Research in Otolaryngology: JARO 2011;12(6):741-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klemm 2007
- Klemm E, Bepperling F, Burschka MA, Mösges R, Study Group. Hemodilution therapy with hydroxyethyl starch solution (130/0.4) in unilateral idiopathic sudden sensorineural hearing loss: a dose-finding, double-blind, placebo-controlled, international multicenter trial with 210 patients. Otology & Neurotology 2007;28(2):157-70. [DOI] [PubMed] [Google Scholar]
Klemm 2009
- Klemm E, Deutscher A, Mosges R. A present investigation of the epidemiology in idiopathic sudden sensorineural hearing loss. Laryngo- Rhino- Otologie 2009;88(8):524-7. [DOI] [PubMed] [Google Scholar]
Kollmeier 2015
- Kollmeier B, Warzybok A, Hochmuth S, Zokoll MA, Uslar V, Brand T, et al. The multilingual matrix test: Principles, applications, and comparison across languages: a review. International journal of audiology 2015;54 Suppl 2:3-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Labus 2010
- Labus J, Breil J, Stutzer H, Michel O. Meta-analysis for the effect of medical therapy vs. placebo on recovery of idiopathic sudden hearing loss. Laryngoscope 2010;120(9):1863-71. [DOI] [PubMed] [Google Scholar]
Lai 2017
- Lai D, Zhao F, Jalal N, Zheng Y. Intratympanic glucocorticosteroid therapy for idiopathic sudden hearing loss: meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96(50):e8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavigne 2016
- Lavigne P, Lavigne F, Saliba I. Intratympanic corticosteroids injections: a systematic review of literature. European Archives of Oto-rhino-laryngology 2016;273(9):2271-8. [DOI] [PubMed] [Google Scholar]
Lawrence 2015
- Lawrence R, Thevasagayam R. Controversies in the management of sudden sensorineural hearing loss: an evidence-based review. Clinical Otolaryngology 2015;40(3):176-82. [DOI] [PubMed] [Google Scholar]
Li 2015
- Li H, Feng G, Wang H, Feng Y. Intratympanic steroid therapy as a salvage treatment for sudden sensorineural hearing loss after failure of conventional therapy: a meta-analysis of randomized, controlled trials. Clinical Therapeutics 2015;37(1):178-87. [DOI] [PubMed] [Google Scholar]
Li 2020
- Li J, Ding L. Effectiveness of steroid treatment for sudden sensorineural hearing loss: a meta-analysis of randomized controlled trials. Annals of Pharmacotherapy 2020;54(10):949-57. [DOI] [PubMed] [Google Scholar]
Liebau 2017
- Liebau A, Pogorzelski O, Salt AN, Plontke SK. Hearing changes after intratympanically applied steroids for primary therapy of sudden hearing loss: a meta-analysis using mathematical simulations of drug delivery protocols. Otology & Neurotology 2017;38(1):19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liebau 2018
- Liebau A, Pogorzelski O, Salt AN, Plontke SK. Hearing changes after intratympanic steroids for secondary (salvage) therapy of sudden hearing loss: a meta-analysis using mathematical simulations of drug delivery protocols. Otology & Neurotology 2018;39(7):803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacArthur 2015
- MacArthur C, Hausman F, Kempton B, Trune DR. Intratympanic steroid treatments may improve hearing via ion homeostasis alterations and not immune suppression. Otology & Neurotology 2015;36(6):1089-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mäder 2018
- Mäder K, Lehner E, Liebau A, Plontke SK. Controlled drug release to the inner ear: concepts, materials, mechanisms, and performance. Hearing Research 2018;368:49-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mao 2005
- Mao HJ, Lu ZM, Zahng HR. Comparison of the effects of two different administration routes with hormone on sudden hearing loss. Chinese Journal of Misdiagnostics 2005;5(12):2203-4. [Google Scholar]
Marx 2018
- Marx M, Younes E, Chandrasekhar SS, Ito J, Plontke S, O'Leary S, et al. International consensus (ICON) on treatment of sudden sensorineural hearing loss. European Annals of Otorhinolaryngology, Head and Neck Diseases 2018;135(1S):S23-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Merchant 2005
- Merchant SN, Adams JC, Nadol JB Jr. Pathology and pathophysiology of idiopathic sudden sensorineural hearing loss. Otology & Neurotology 2005;26(2):151-60. [DOI] [PubMed] [Google Scholar]
Merchant 2008
- Merchant SN, Durand ML, Adams JC. Sudden deafness: is it viral? ORL: Journal of Oto-Rhino-Laryngology and its Related Specialties 2008;70(1):52-60; discussion 60-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirian 2020
- Mirian C, Ovesen T. Intratympanic vs systemic corticosteroids in first-line treatment of idiopathic sudden sensorineural hearing loss: a systematic review and meta-analysis. JAMA Otolaryngology--Head & Neck Surgery 2020;146(5):421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mösges 2009
- Mösges R, Köberlein J, Heibges A, Erdtracht B, Klingel R, Lehmacher W, RHEO-ISHL Study Group. Rheopheresis for idiopathic sudden hearing loss: results from a large prospective, multicenter, randomized, controlled clinical trial. European Archives of Oto-Rhino-Laryngology 2009;266(7):943-53. [DOI] [PubMed] [Google Scholar]
Nakagawa 2011
- Nakagawa T, Ito J. Local drug delivery to the inner ear using biodegradable materials. Therapeutic Delivery 2011;2(6):807-14. [DOI] [PubMed] [Google Scholar]
Ng 2015
- Ng JH, Ho RC, Cheong CS, Ng A, Yuen HW, Ngo RY. Intratympanic steroids as a salvage treatment for sudden sensorineural hearing loss? A meta-analysis. European Archives of Oto-rhino-laryngology 2015;272(10):2777-82. [DOI] [PubMed] [Google Scholar]
NIDCD 2018
- NIDCD. National Institute on Deafness and Other Communication Disorders. NIDCD Fact Sheet: Sudden Deafness. Washington, DC: US Department of Health and Human Services; 2018. https://www.nidcd.nih.gov (accessed 20 April 2022).
Niedermeyer 2003
- Niedermeyer HP, Zahneisen G, Luppa P, Busch R, Arnold W. Cortisol levels in the human perilymph after intravenous administration of prednisolone. Audiology & Neuro-otology 2003;8(6):316-21. [DOI] [PubMed] [Google Scholar]
Nosrati‐Zarenoe 2012
- Nosrati-Zarenoe R, Hultcrantz E. Corticosteroid treatment of idiopathic sudden sensorineural hearing loss: randomized triple-blind placebo-controlled trial. Otology & Neurotology 2012;33(4):523-31. [DOI] [PubMed] [Google Scholar]
Olzowy 2005
- Olzowy B, Osterkorn D, Suckfüll M. The incidence of sudden hearing loss is greater than previously assumed [German]. MMW Fortschritte der Medizin 2005;147(14):37-8. [PubMed] [Google Scholar]
Pararas 2012
- Pararas EE, Borkholder DA, Borenstein JT. Microsystems technologies for drug delivery to the inner ear. Advanced Drug Delivery Reviews 2012;64(14):1650-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parnes 1999
- Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope 1999;109(7 Pt 2):1-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Phillips 2011
- Phillips JS, Westerberg B. Intratympanic steroids for Meniere's disease or syndrome. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD008514. [DOI: 10.1002/14651858.CD008514.pub2] [DOI] [PubMed] [Google Scholar]
Plaza 2007
- Plaza G, Herráiz C. Intratympanic steroids for treatment of sudden hearing loss after failure of intravenous therapy. Otolaryngology - Head & Neck Surgery 2007;137(1):74-8. [DOI] [PubMed] [Google Scholar]
Plontke 2005
- Plontke S. Therapy of hearing disorders - conservative procedures. In: Beleites E, Gudziol H, editors(s). Restoring Methods of Functional Defects in Head and Neck. 1st edition. Köln, Germany: Scientias Verlag, 2005:1-65. [PMC free article] [PubMed] [Google Scholar]
Plontke 2007
- Plontke SK, Bauer M, Meisner C. Comparison of pure tone audiometry analysis in sudden hearing loss studies: lack of agreement for different outcome measures. Otology & Neurotology 2007;28(6):753-63. [DOI] [PubMed] [Google Scholar]
Plontke 2016
- Plontke SK, Girndt M, Meisner C, Probst R, Oerlecke I, Richter M, et al. Multicenter trial for sudden hearing loss therapy - planning and concept [Multizentrische Studie zur Hörsturztherapie - Planung und Konzeption]. HNO 2016;64(4):227-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Probst 1992
- Probst R, Tschopp K, Lüdin E, Kellerhals B, Podvinec M, Pfaltz CR. A randomized, double-blind, placebo-controlled study of dextran/pentoxifylline medication in acute acoustic trauma and sudden hearing loss. Acta Oto-Laryngologica 1992;112(3):435-43. [DOI] [PubMed] [Google Scholar]
Quaranta 2016
- Quaranta N, De Ceglie V, D'Elia A. Endothelial dysfunction in idiopathic sudden sensorineural hearing loss: a review. Audiology Research 2016;6(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quiang 2016
- Quiang Q, Wu X, Yang T, Yang C, Sun H. A comparison between systemic and intratympanic steroid therapies as initial therapy for idiopathic sudden sensorineural hearing loss: a meta-analysis. Acta Oto-laryngologica 2017;137(6):598-605. [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rohrmeier 2012
- Rohrmeier C, Koemm N, Babilas P, Prahs P, Strutz J, Buettner R. Sudden sensorineural hearing loss: systemic steroid therapy and the risk of glucocorticoid-induced hyperglycemia. European Archives of Oto-rhino-laryngology 2012;270(4):1255-61. [DOI] [PubMed] [Google Scholar]
Salt 2009
- Salt AN, Plontke SK. Principles of local drug delivery to the inner ear. Audiology & Neuro-otology 2009;14(6):350-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salt 2018
- Salt AN, Plontke SK. Pharmacokinetic principles in the inner ear: influence of drug properties on intratympanic applications. Hearing Research 2018;368:28-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salt 2020
- Salt AN, Plontke SK. Steroid nomenclature in inner ear therapy. Otology & Neurotology 2020;41(6):722-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11:32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seggas 2011
- Seggas I, Koltsidopoulos P, Bibas A, Tzonou A, Sismanis A. Intratympanic steroid therapy for sudden hearing loss: a review of the literature. Otology & Neurotology 2011;32(1):29-35. [DOI] [PubMed] [Google Scholar]
She 2010
- She W, Dai Y, Du X, Yu C, Chen F, Wang J, et al. Hearing evaluation of intratympanic methylprednisolone perfusion for refractory sudden sensorineural hearing loss. Otolaryngology - Head & Neck Surgery 2010;142(2):266-71. [DOI] [PubMed] [Google Scholar]
Siegel 1975
- Siegel LG. The treatment of idiopathic sudden sensorineural hearing loss. Otolaryngologic Clinics of North America 1975;8(2):467-73. [PubMed] [Google Scholar]
Silverstein 1996
- Silverstein H, Choo D, Rosenberg SI, Kuhn J, Seidman M, Stein I. Intratympanic steroid treatment of inner ear disease and tinnitus (preliminary report). Ear, Nose, and Throat Journal 1996;75(8):468-71, 474, 476 passim. [PubMed] [Google Scholar]
Spear 2011
- Spear SA, Schwartz SR. Intratympanic steroids for sudden sensorineural hearing loss: a systematic review. Otolaryngology - Head and Neck Surgery 2011;145(4):534-43. [DOI] [PubMed] [Google Scholar]
Stachler 2012
- Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngology - Head and Neck Surgery 2012;146(3 Suppl):1-35. [DOI] [PubMed] [Google Scholar]
Stokroos 1996
- Stokroos RJ, Albers FW. Therapy of idiopathic sudden sensorineural hearing loss. A review of the literature. Acta Oto-Rhino-Laryngologica Belgica 1996;50(1):77-84. [PubMed] [Google Scholar]
Suckfuell 2014
- Suckfuell M, Lisowska G, Domka W, Kabacinska A, Morawski K, Bodlaj R, et al. Efficacy and safety of AM-111 in the treatment of acute sensorineural hearing loss: a double-blind, randomized, placebo-controlled phase II study. Otology & Neurotology 2014;35(8):1317-26. [DOI] [PubMed] [Google Scholar]
Suckfüll 2002
- Suckfüll M, Hearing Loss Study Group. Fibrinogen and LDL apheresis in treatment of sudden hearing loss: a randomised multicentre trial. Lancet 2002;360(9348):1811-7. [DOI] [PubMed] [Google Scholar]
Tran Ba Huy 2005
- Tran Ba Huy PT, Sauvaget E. Idiopathic sudden sensorineural hearing loss is not an otologic emergency. Otology & Neurotology 2005;26(5):896-902. [DOI] [PubMed] [Google Scholar]
Trune 2006
- Trune DR, Kempton JB, Gross ND. Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hearing Research 2006;212(1-2):22-32. [DOI] [PubMed] [Google Scholar]
Trune 2012
- Trune DR, Canlon B. Corticosteroid therapy for hearing and balance disorders. Anatomical Record (Hoboken, N.J.: 2007) 2012;295(11):1928-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tucci 2002
- Tucci DL, Farmer JC Jr, Kitch RD, Witsell DL. Treatment of sudden sensorineural hearing loss with systemic steroids and valacyclovir. Otology & Neurotology 2002;23(3):301-8. [DOI] [PubMed] [Google Scholar]
Vambutas 2021
- Vambutas A, Davia DV. Biologics for immune-mediated sensorineural hearing loss. Otolaryngologic clinics of North America 2021;54(4):803-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ventry 1982
- Ventry IM, Weinstein BE. The hearing handicap inventory for the elderly: a new tool. Ear and Hearing 1982;3(3):128-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vlastarakos 2012
- Vlastarakos PV, Papacharalampous G, Maragoudakis P, Kampessis G, Maroudias N, Candiloros D, et al. Are intra-tympanically administered steroids effective in patients with sudden deafness? Implications for current clinical practice. European Archives of Oto-rhino-laryngology 2012;269(2):363-80. [DOI] [PubMed] [Google Scholar]
Wei 2006
- Wei BPC, Mubiru S, O'Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No: CD003998. [DOI: 10.1002/14651858.CD003998.pub2] [DOI] [PubMed] [Google Scholar]
Wei 2013
- Wei BP, Stathopoulos D, O'Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD003998. [DOI: 10.1002/14651858.CD003998.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1980
- Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Archives of Otolaryngology 1980;106(12):772-6. [DOI] [PubMed] [Google Scholar]
Wycherly 2011
- Wycherly BJ, Thompkins JJ, Jeffrey Kim H. Early posttreatment audiometry underestimates hearing recovery after intratympanic steroid treatment of sudden sensorineural hearing loss. International Journal of Otolaryngology 2011;2011:465831. [DOI: 10.1155/2011/465831] [DOI] [PMC free article] [PubMed]
Yi 2011
- Yi JC, Chen S, Huang CY. Observation on the therapeutic effect of Eustachian tube injection of dexamethasone in treatment of sudden deafness. Guangxi Medical Journal 2011;33(2):179-80. [Google Scholar]
You 2008
- You XL, Lin X, Zhu BC. Local and systemic glucocorticoid treatment for sudden hearing loss. China Practical Medical 2008;3(28):125-7. [Google Scholar]
Zhang 2021
- Zhang Z, Li X, Zhang W, Kohane DS. Drug delivery across barriers to the middle and inner ear. Advanced Functional Materials 2021;31(44):2008701. [DOI: 10.1002/adfm.202008701] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2016
- Zhao D, Tong B, Wang Q, Hellstrom S, Duan M. A comparison of effects of systemic and intratympanic steroid therapies for sudden sensorineural hearing loss: a meta-analysis. Journal of Otology 2016;11:18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Plontke 2009
- Plontke SK, Meisner C, Caye‐Thomasen P, Parnes L, Agrawal S, Mikulec T. Intratympanic glucocorticoids for sudden sensorineural hearing loss. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD008080. [DOI: 10.1002/14651858.CD008080] [DOI] [PMC free article] [PubMed] [Google Scholar]


