Figure 3: No microglial or astrocytic changes associated with microglial-specific ApoE knock-out.
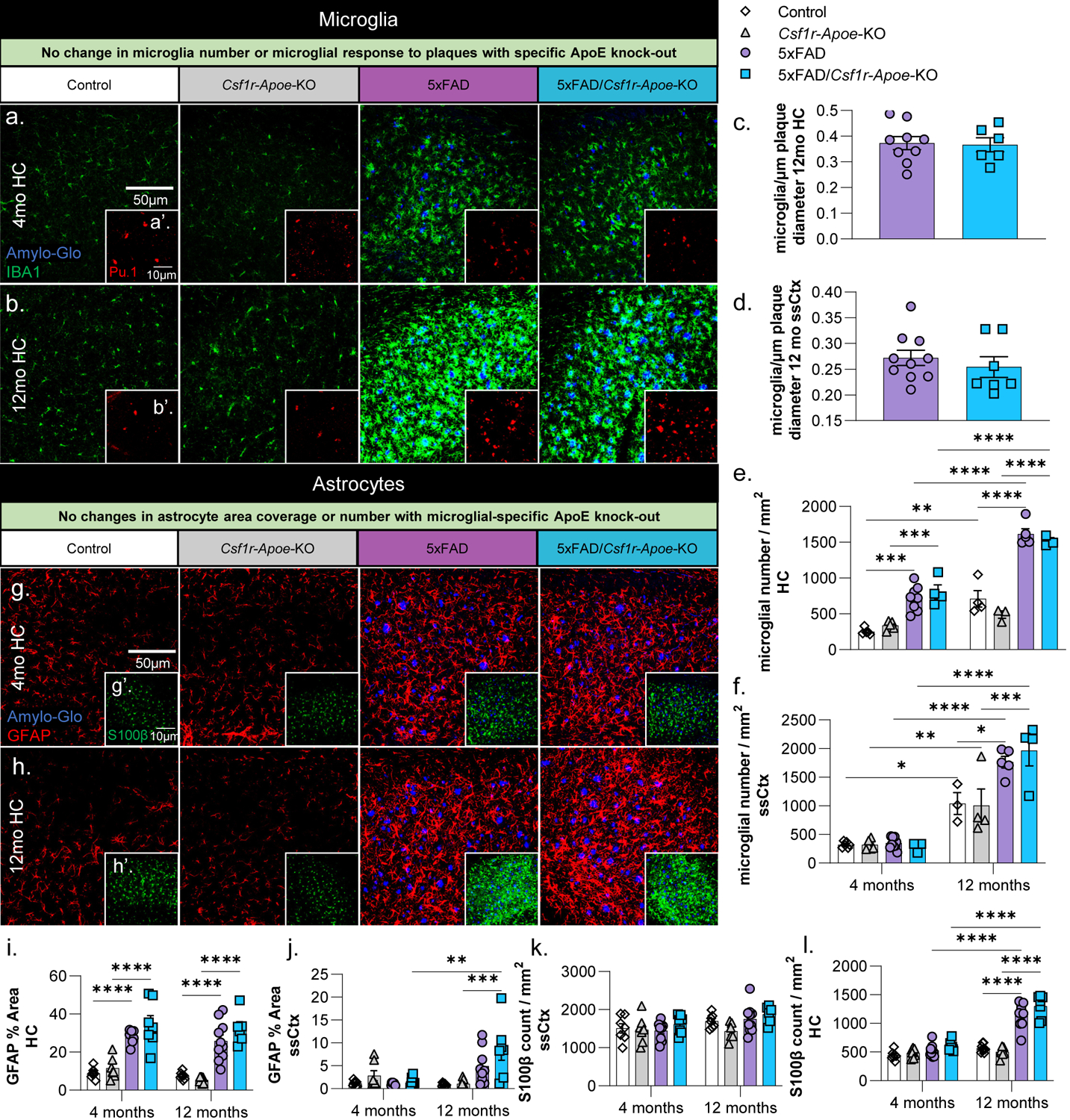
Representative 20x images of Amylo-Glo dye staining and IBA1 (microglia) immunofluorescence in Control, Csf1r-Apoe-KO, 5xFAD, and 5xFAD/Csf1r-Apoe-KO mice at 4- (a) and 12-months (b) of age in the hippocampus. Additional Amylo-Glo staining and Pu.1 (microglia) immunofluorescence images of all groups at 4- (a’) and 12-months (b’) of age in the hippocampus. No difference in the number of microglia/plaque diameter (μm) was observed between 5xFAD (n=9) and 5xFAD/Csf1r-Apoe-KO (n=7) mice in the hippocampus (c) nor somatosensory cortex (d). Microglial number between Control and 5xFAD mice is increased at 12-months of age in but no differences in microglial number were seen between Control (4 months n=5; 12 months n=4) and Csf1r-Apoe-KO (4 months n=5; 12 months n=3) or 5xFAD (4 months n=8; 12 months n=5) and 5xFAD/Csf1r-Apoe-KO (4 months n=4; 12 months n=4) mice in the hippocampus (e) nor somatosensory cortex (f). Representative 20x images of Amylo-Glo dye staining and GFAP (reactive astrocytes) immunofluorescence in all four groups at 4- (g) and 12-months (h) of age in the hippocampus. Additional Amylo-Glo staining and S100β (astrocyte cell bodies) immunofluorescence images of all groups at 4- (g’) and 12-months (h’) of age in the hippocampus. We observed significant increases in GFAP percent area quantifications in 5xFAD (4 months n=8; 12 months n=10) and 5xFAD/Csf1r-Apoe-KO (4 months n=7; 12 months n=6) groups compared to the Control (4 months n=7; 12 months n=8) and Csf1r-Apoe-KO (4 months n=7; 12 months n=7) in the hippocampus (i) at 4 and 12 months of age, and in the somatosensory cortex (j) at 12 months of age. In both the hippocampus and somatosensory cortex, no differences in astrocyte percent area were seen between Control and Csf1r-Apoe-KO groups or 5xFAD and 5xFAD/Csf1r-Apoe-KO groups. S100β astrocyte counts show no differences between Control and Csf1r-Apoe-KO groups or 5xFAD and 5xFAD/Csf1r-Apoe-KO groups in the hippocampus (k) or somatosensory cortex (l). Statistical analysis used a two-way ANOVA with Tukey’s multiple comparisons correction and a two-tailed t-test for the microglia/plaque diameter (μm) quantification. Significance indicated as * p< 0.05; ** p < 0.01; *** p < 0.001; # 0.05 < p < 0.1.
