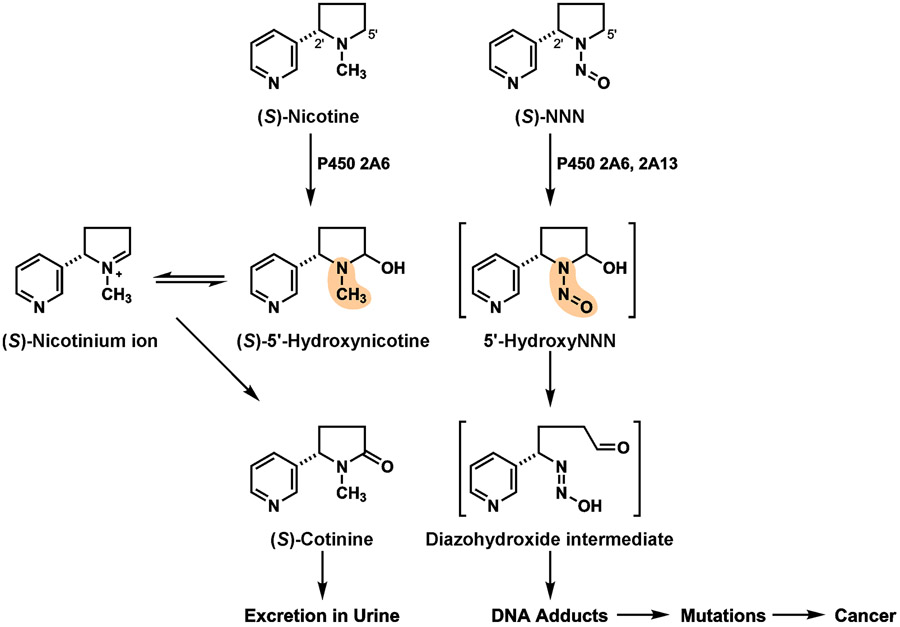
Figure 3 ∣. Metabolism of (S)-nicotine and (S)-NNN by 5'-hydroxylation.
Metabolism of (S)-nicotine and (S)-NNN by this pathway are similar, yet lead to drastically different results. The red atoms in the structures are the different functional groups that explain the results. For NNN, after 5'-hydroxylation by CYP2A6 or CYP2A13, the properties of the N-N=O group in 5'-hydroxyNNN cause immediate transformation to the DNA damaging diazohydroxide intermediate illustrated, leading to DNA adducts and cancer. In contrast, the highlighted N-CH3 group of nicotine does not have this effect, but rather is further oxidized to the innocuous compound (S)-cotinine, which is stable and is excreted in urine. (Brackets indicate unstable intermediates).