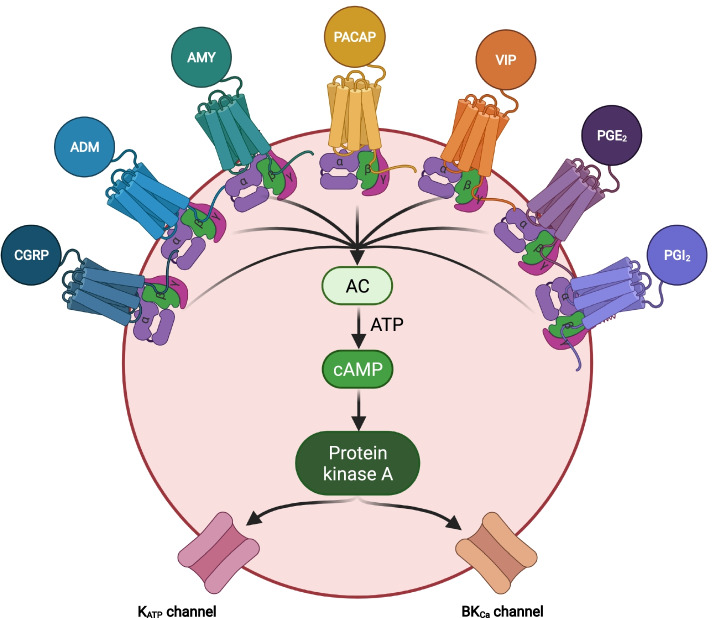
Fig. 1.
cAMP-dependent pathways in migraine pathophysiology. The cell is a vascular smooth muscle cell within the walls of intracranial arteries. Experimental studies have shown that binding of calcitonin gene-related peptide (CGRP), adrenomedullin (ADM), amylin (AMY), Pituitary adenylate cyclase-activating polypeptide (PACAP), vasoactive intestinal polypeptide (VIP), prostaglandin E2 (PGE2), and prostaglandin I2 (PGI2) to their G protein-coupled receptors increases the intracellular concentration of cyclic adenosine monophosphate (cAMP) and thereby activates the cAMP-dependent pathway. This will then activate protein kinase A which, in turn, results in outflow of potassium via opening of adenosine triphosphate-sensitive potassium (KATP) channels and large conductance calcium-activated potassium (BKCa) channels. The end result is hyperpolarization of the vascular smooth muscle and accompanying vasodilation which is hypothesized to provide the necessary chemical and mechanical stimuli needed to activate and sensitize perivascular nociceptors [2]. AC, adenylate cyclase; ADM, adrenomedullin; AMY, amylin; ATP, adenosine triphosphate; BKCA, large conductance calcium-activated potassium channels; cAMP, cyclic adenosine monophosphate; CGRP, calcitonin-gene related peptide; KATP-channels, adenosine triphosphate-sensitive potassium channels; PACAP, pituitary adenylate cyclase activating polypeptide; PGE2, prostaglandin E2; PGI2, prostaglandin I2; Protein kinase A, cAMP-dependent protein kinase; VIP, vasoactive intestinal polypeptide