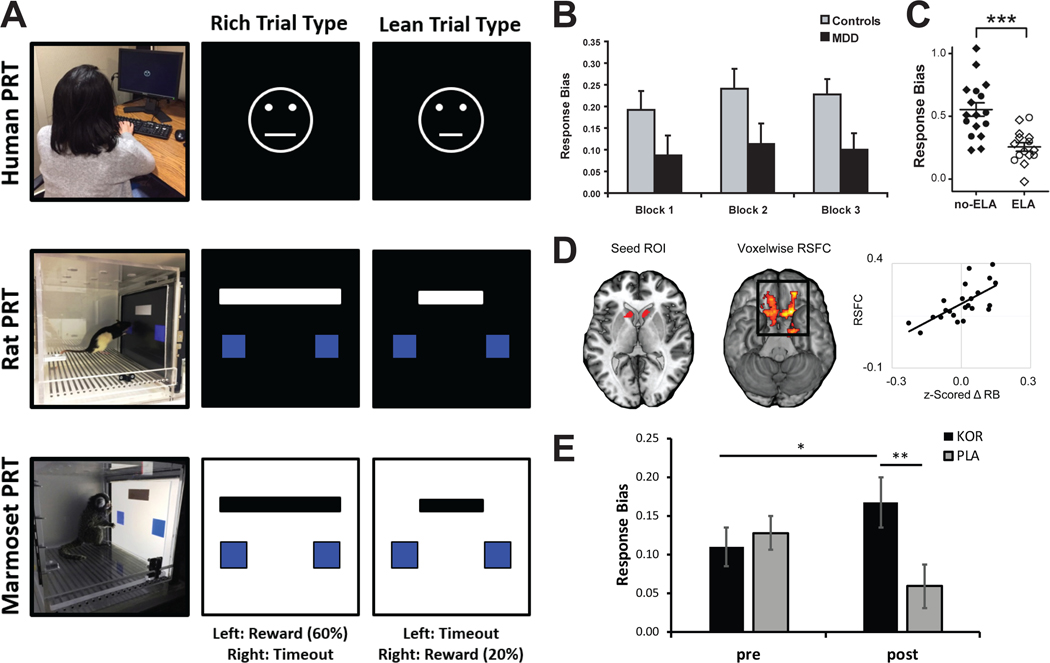
Figure 3:
Cross-species Reward Learning Assay. (A) Task schematics for the human (top; (108)), rat (middle; (110)), and marmoset (bottom; (109)) Probabilistic Reward Task (PRT). Using a signal-detection approach involving an asymmetric reinforcement schedule and two difficult-to-discriminate stimuli, the PRT objectively assesses subjects’ ability to develop a response bias toward a more frequently rewarded stimulus, which is taken as a measure of reward learning. (B) Relative to healthy controls, unmedicated individuals with major depressive disorder (MDD) have significantly lower response bias (117). (C) Relative to a no-stress (control) group, rats exposed to early life stress (limited bedding and nesting paradigm) between P2 and P9 were characterized by signiciantly blunted response bias (118). (D) Among healthy controls, individual differences in response bias are positively associated with resting state functional connectivity between the ventral striatum and the ventromedial prefrontal cortex (as assessed with fMRI) and negatively associated with dopamine transporter binding potential (not shown; as assessed using positron emission tomography). The latter finding suggests that low dopamine transporter availability, and thus higher dopamine availability in the synaptic cleft, is associated with better reward learning abilities (119). (E) In a placebo-controlled clinical trial, a kappa opioid antagonist was associated with better response bias among a transdiagnostic sample with elevated anhedonia (87). Figures reproduced with permissions from the publishers.