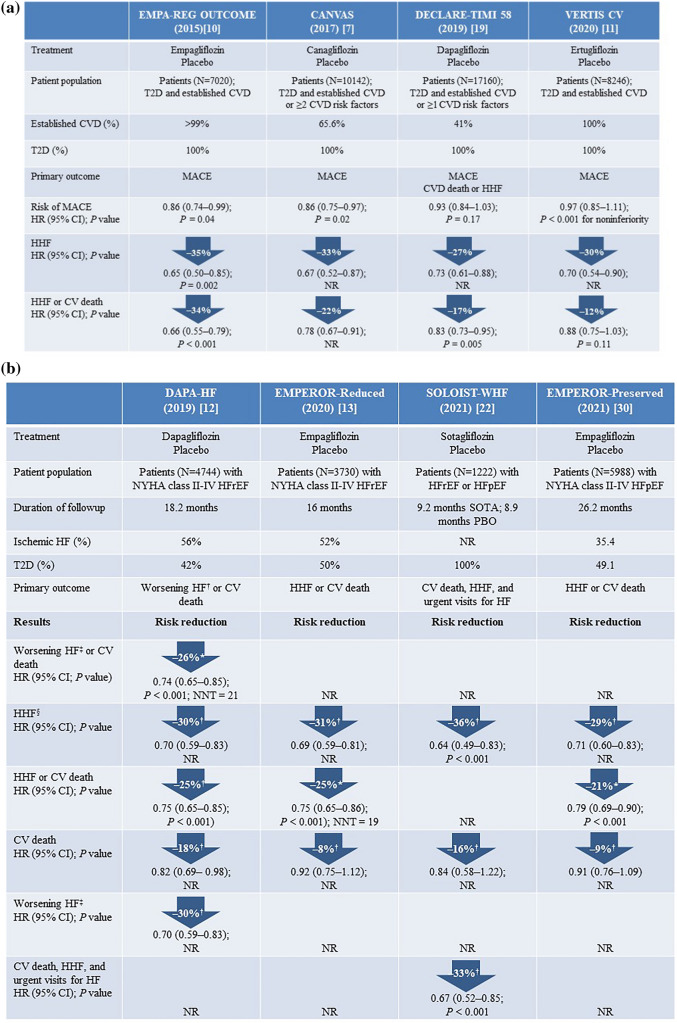
Fig. 1.
Summary of HF-related outcomes observed in trials with SGLT2is in a patients with T2D [7, 10, 11, 19] and b patients with HFrEF [12, 13, 22, 30]. CI confidence interval, CV cardiovascular, CVD cardiovascular disease, HF heart failure, HFpEF HF with preserved ejection fraction, HFrEF HF with reduced ejection fraction, HHF hospitalization for HF, HR hazard ratio, MACE major adverse CV events (CV death, non-fatal myocardial infarction, or non-fatal stroke), NNT number needed to treat, NR not reported, NYHA New York Heart Association, PBO placebo, SGLT2i sodium–glucose cotransporter 2 inhibitor, SOTA sotagliflozin, T2D type 2 diabetes. *Primary study end point; †Secondary or other end points; ‡Defined as an unplanned hospitalization for HF or an urgent visit resulting in intravenous therapy for heart failure; §Defined in SOLOIST-WHF as hospitalizations and urgent visits for HF