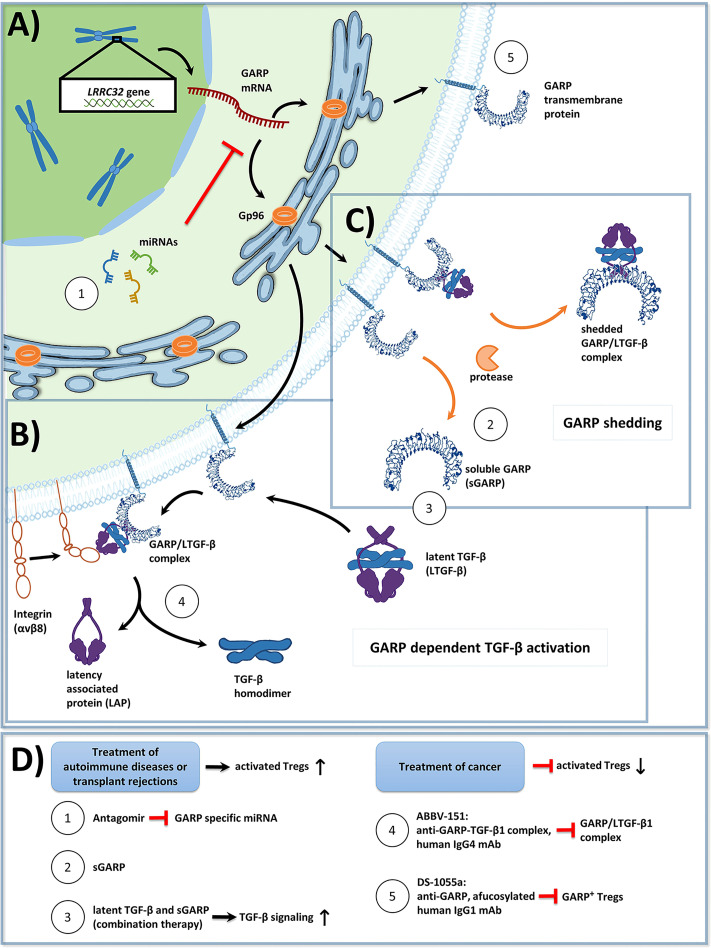
Figure 1.
(A) Overview of Glycoprotein a repetitions predominant (GARP) protein biosynthesis and transport to the cell membrane in activated human regulatory T cells (Tregs). GP96, a chaperone found in the endoplasmic reticulum, ensures proper folding of GARP [GARP structure modified from: Liénart et al. (35)]. The GARP mRNA is targeted by miRNA, which promote its degradation, and thus lower GARP mRNA/GARP levels. (B) GARP functions as a docking receptor for biologically inactive latent transforming growth factor beta (LTGF-β), which consists of a TGF-β homodimer bound to latency associated protein (LAP), and GARP plays an important role in its activation. GARP binds LTGF-β with high affinity, forming the GARP/LTGF-β complex. Release of bioactive TGF-β can occur in both a protease independent (shown) or a protease dependent manner (not shown). For protease independent release of mature TGF-β, αVβ8 integrins, expressed on the surface of Treg bind to the GARP/LTGF-β complex, resulting in a conformational change and in the subsequent release of biologically active TGF-β. Alternatively, bioactive TGF-β can be released in a protease dependent manner, in which integrin recruited metalloproteinases or serine proteases cleave LAP from the GARP/LTGF-β complex (not shown). (C) GARP can be cleaved from the surface of Treg by proteases in a form called soluble GARP (sGARP). GARP/LTGF-β complexes can also be released into the extracellular environment via proteases. (D) Potential methods to target GARP for the treatment of autoimmune diseases, transplant rejections, and cancer. Enhancing GARP mediated suppression by Treg offers a promising strategy for the treatment of autoimmune diseases and transplant rejections. (1) Approach 1 utilizes antagomirs, specific to miRNA, which target the GARP mRNA. This would prevent GARP mRNA degradation, and thus increase surface GARP expression and enhance the suppressive capacity of Treg. (2) Approach 2 is to apply sGARP to induce Treg. (3) Approach 3 would be to apply sGARP in combination with LTGF-β to harness both their immunosuppressive effects and to promote the activation of TGF-β in an integrin-controlled manner. For effective anti-tumor immune responses to occur, Treg mediated suppression needs to be inhibited. (4-5) Approaches 4 and 5 represent two different monoclonal antibody (mAb) therapeutic strategies, that are currently in phase 1 clinical trials. (4) Approach 4 uses an IgG4 antibody (ABBV-151) that binds to the GARP/TGF-β1 complex and prevents the release of mature TGF-β1. This results in an inhibition of TGF-β1 signaling, a subsequent decrease in the suppressive capacity of Treg, and the restoration of T effector cell (Teff) functions. (5) Approach 5 employs an afucosylated IgG1 antibody (DS-1055a), that efficiently depletes GARP+ Treg via antibody-dependent cellular cytotoxicity, preventing Treg mediated suppression and restoring Teff function.