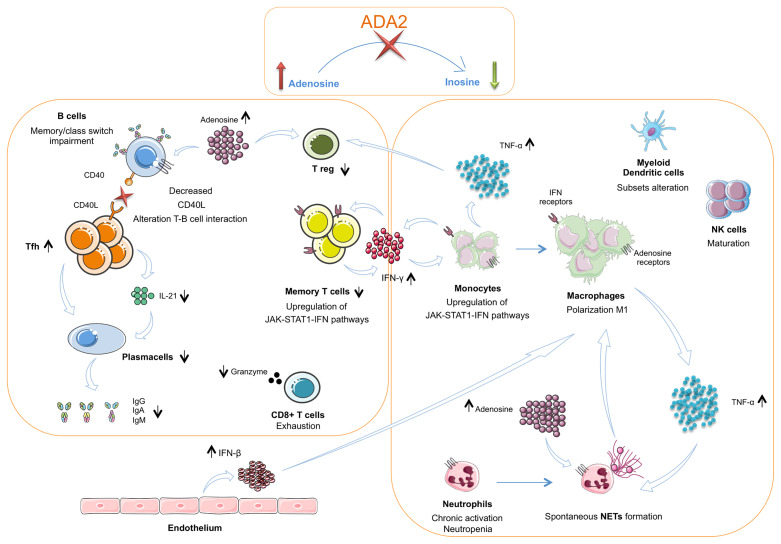
Figure 2.
The interplay between innate and adaptive immune cells in DADA2. The insufficient or absent ADA2 enzymatic activity leads to a reduction of adenosine conversion into inosine and to an accumulation of adenosine in the extracellular space. In the innate immune system this leads to a chronic neutrophils activation and NETosis dysregulation with spontaneous NETs formation. Macrophages show a polarization towards the M1 subtype with consequent hypersecretion of inflammatory cytokines, especially TNF-α Dysregulation of NETosis and IFN-β secreted in large amount by endothelium also contribute to the M1 polarization. TNF-α acts on neutrophils contributing to maintaining their chronic activation state. CD56bright immature NK cells are increased. Dendritic cells present an altered subsets distribution. TNF-α, together with adenosine, acts also on Treg cells, that result diminished. In the T cell compartment, Tfh cells show an increased frequency but an impaired secretion of IL-21, crucial for B-cell function. Furthermore, T cells present a reduced proportion of the memory subsets and an alteration of CD40L expression. This defect is associated with an impaired B cell proliferation, differentiation and switching with consequent lower levels of immunoglobulins. CD8+ T cells show an exhausted phenotype and produce low amounts of granzyme A. Both T cells and monocytes show an upregulation of JAK – STAT1 – IFN pathways. In monocytes this activation contributes to the M1 macrophage polarization. TNF-α, Tumor necrosis factor α; IFN β, Interferon β; NETs, Neutrophils extracellular traps.