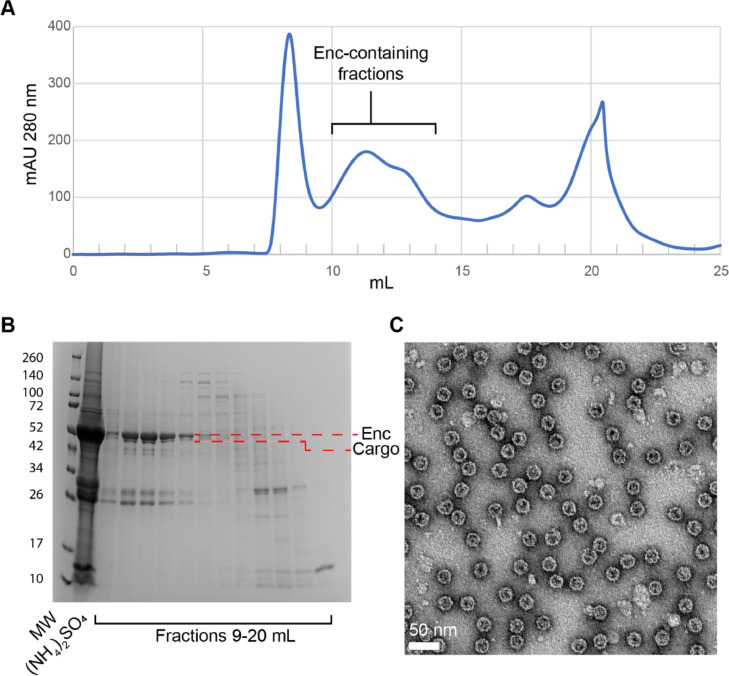
Fig. 5.
Successful purification of an encapsulin from S. coelicolor. (A) Size exclusion chromatography (Superose 6 Increase 10/300 GL) of an encapsulin (Q54255) and its cargo protein (A0A2 × 2LWM5) according to Section E. T1 encapsulin samples generally elute between 10 and 13 mL. Enc: encapsulin. (B) SDS-PAGE gel of the encapsulin sample purified in panel A. Enc and the cargo protein bands were unambiguously identified via tryptic digest and mass spectrometry identification. The bands around the 26 and 34 kDa marker bands represent the following often co-purifying ribosomal proteins as identified via tryptic digest and mass spectrometry: TsnR family 23S rRNA methyltransferase, 29 kDa (A0A6M9XPU2), 30S ribosomal protein S3, 30 kDa (Q9L0D4), 30S ribosomal protein S4, 24 kDa (Q9KXP5). The encapsulin protein is most enriched in fractions 10–12 and is less enriched in fraction 13. MW: molecular weight marker, (NH4)2SO4: ammonium sulfate pellet. (C) Negative stain TEM micrograph of fraction 10 from panels A and B. The micrograph was collected using an FEI Morgagni 100 kV electron microscope. The sample concentration: 0.1 mg/mL, stain: 0.2% uranyl formate, grid: freshly glow-discharged formvar-reinforced carbon grid (EMS, FCF200-AU-EC).