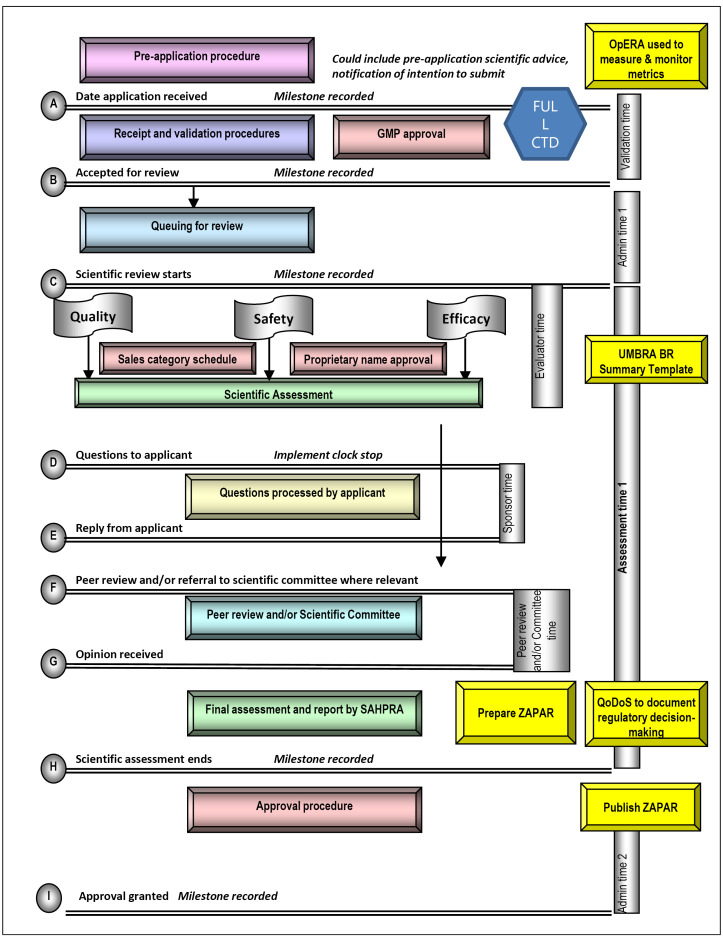
Figure 1.
Proposed Model for the Improved Review Process. Adapted from Keyter et al 7 and Keyter et al. 17 Abbreviations: BR, benefit-risk; CTD, common technical document; GMP, good manufacturing practice; OpERA, optimising efficiencies in regulatory agencies; SAHPRA, South African Health Products Regulatory Authority; QoDoS, quality of decision-making orientation scheme; UMBRA,universal methodologies for benefit-risk assessment; ZAPAR, South African Public Assessment Report.