Fig. 3 |. Homeostatic regulation of SPT mediated by ORMDL proteins.
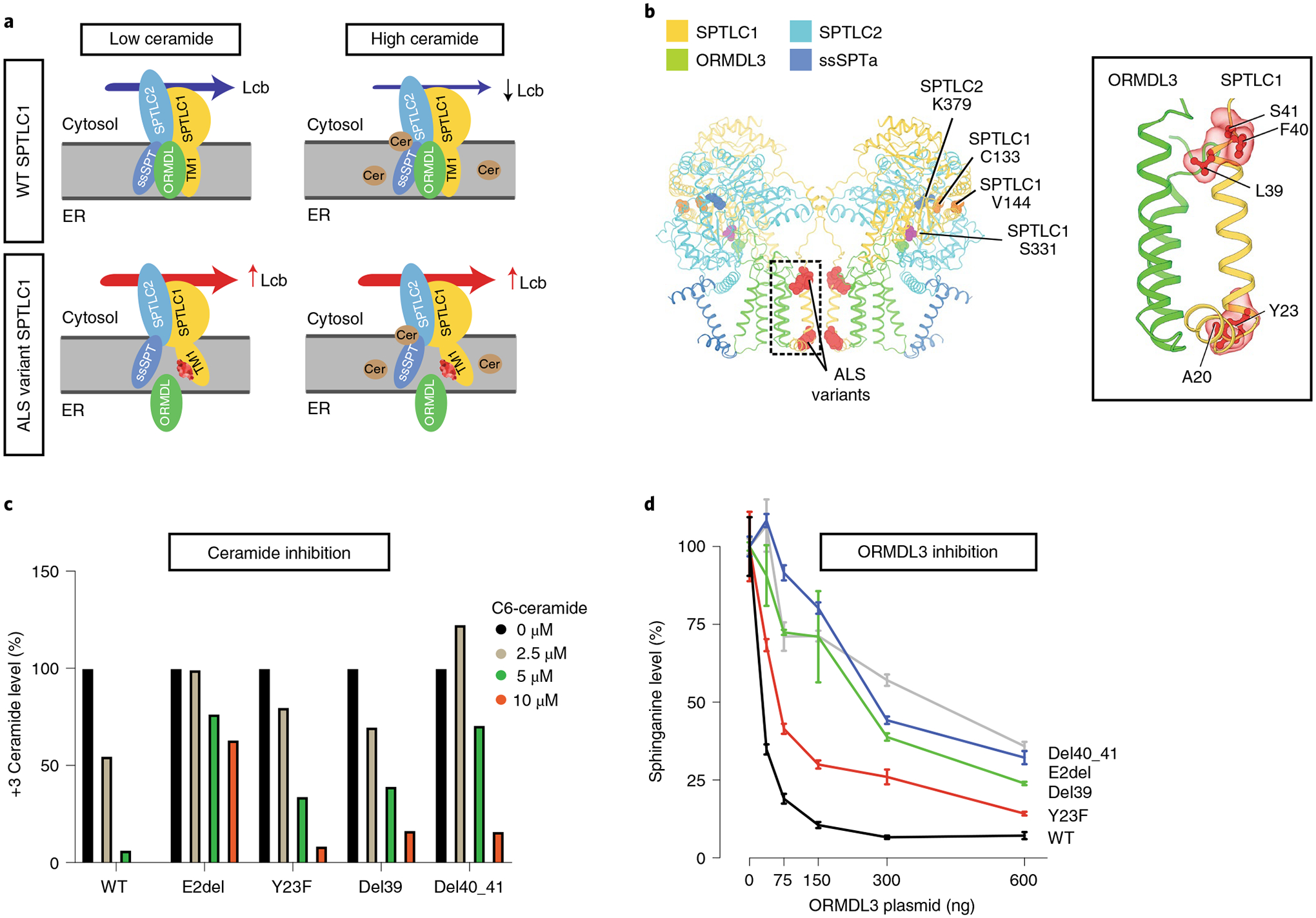
a, The SPT multisubunit complex includes SPTLC1, SPTLC2 and a small subunit (ssSPT). ORMDL proteins bind the first transmembrane domain of SPTLC1 (TM1) and inhibit SPT activity in the presence of high ceramide (Cer) levels. SPTLC1 with ALS variants in TM1 does not bind ORMDLs efficiently, and high ceramide levels are less effective in inhibition of mutant SPT activity. Lcb, long-chain base; ER, endoplasmic reticulum. b, Cryoelectron microscopy structure of the SPT/ORMDL3/ssSPTa complex. Hereditary sensory neuropathy SPTLC1 residues (for example, C133 and V144) are in the cytosolic portion of SPT, near its active site and the pyridoxal phosphate-binding residue of SPTLC2 (K379). In contrast, ALS-associated variants are near the ORMDL3-interacting transmembrane domain (inset). c, HEK293 SPTLC1 knockout cells transfected with WT or ALS-associated SPTLC1 variants. De novo synthesized ceramides (labeled with D3-15N-l-serine and D4-l-alanine) were measured in the presence of increasing, exogenously added C6-ceramide. Bar graphs show normalized +3 ceramides for each cell line. d, WT HEK293 cells were cotransfected with SPTLC2, ssSPTa, either WT or ALS variant SPTLC1 and increasing amounts of ORMDL3 plasmid. SPT activity was evaluated by measurement of sphinganine levels normalized to baseline for each cell line. Increasing ORMDL3 has a more robust inhibitory effect on sphinganine synthesis (that is, SPT activity) in WT versus ALS-associated SPTLC1 variants. Error bars are s.e.m. from five independent replicates.
