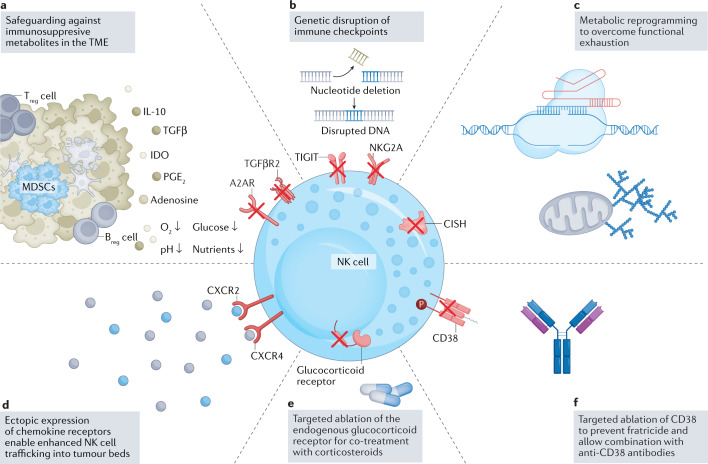
Fig. 4. Genetic engineering strategies to overcome suppressors of NK cell function.
a–f | Immune cell function is severely compromised by the hostile tumour microenvironment (TME)134. Current strategies leverage engineering tools to disrupt suppressive signals in the TME (panel a) and improve immune cell homing into tumour beds by ectopic expression of chemokine receptors (panel d). A selection of natural killer (NK) cell-relevant pathways that have been targeted through genetic engineering is shown. Genetic engineering strategies that include targeted ablation of inhibitory checkpoints156,164,167,168 (panels b,c) as well as disruption of extracellular receptors which sense inhibitory stimuli including TGFβ140,141 and adenosine135 (panel a) have been shown preclinically to effectively target pathways to enhance metabolic fitness and persistence of NK cells, and efforts are ongoing to advance these findings into the clinic. Ablation of endogenous receptors allows for combinatorial therapeutic approaches, such as by rendering immune cells resistant to corticosteroid-induced immunosuppression (panel e), a principle previously established in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-directed cytotoxic T lymphocytes (CTLs)281. Knockout of CD38 (panel f) renders NK cells resistant to CD38-mediated fratricide, which enables combination strategies of NK cells and anti-CD38-targeting monoclonal antibodies in the context of treating multiple myeloma50. Breg cell, regulatory B cell; MDSC, myeloid-derived suppressor cell; NKG2A, CD94/NK group 2 member A receptor; TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domains; Treg cell, regulatory T cell.